CH391L/S12/TranscriptionPromotersandTerminators: Difference between revisions
| (2 intermediate revisions by the same user not shown) | |||
| Line 22: | Line 22: | ||
====Determining Strength==== | ====Determining Strength==== | ||
The strength of the different promoters is determined by the relative frequency of transcription initiation. This is mainly affected by the affinity of the promoter sequence for RNA polymerase. | The strength of the different promoters is determined by the relative frequency of transcription initiation. This is mainly affected by the affinity of the promoter sequence for RNA polymerase. <cite>#ishihama</cite>Quantitatively measuring this can be done by monitoring protein synthesis rate of a protein such as GFP. Promoters that differ significantly from the consensus sequence will be weaker than those that resemble the consensus sequence due to binding affinity.<cite>Haventsitedyet</cite> | ||
===Promoter Examples=== | ===Promoter Examples=== | ||
| Line 34: | Line 34: | ||
====Constitutive Bacterial Promoter Design==== | ====Constitutive Bacterial Promoter Design==== | ||
[[Image:PromoterDesign.gif|Promoters designed by Saurer et al. compare insulated promoters against minimal promoters<cite>#Saurer2010</cite>|thumb|400px|]] | [[Image:PromoterDesign.gif|Promoters designed by Saurer et al. compare insulated promoters against minimal promoters<cite>#Saurer2010</cite>|thumb|400px|]] | ||
Sauer et al. generate various promoters spanning two orders in magnitude of strength. The promoters were based on the E. coli rrnB P1, a strong σ70-dependent promoter with near consensus −10 (TATAAT) and −35 (TTtACg) elements. The region from position -105 to +55 were insulated, blocking | Sauer et al. generate various promoters spanning two orders in magnitude of strength. The promoters were based on the E. coli rrnB P1, a strong σ70-dependent promoter with near consensus −10 (TATAAT) and −35 (TTtACg) elements. The region from position -105 to +55 were insulated by nucleotide sequences that did not bind proteins, blocking all transcription factor binding sites, enhancer sites, as well as defining a specific 5' mRNA start site. Using proD, their first generation insulated promoter, the production of the GFP reporter gene was driven. Measuring strength of this promoter relative to another, the GFP synthesis rate was monitored. They found that the insulated proD promoter performed significantly better than a minimal promoter. Through the use of degenerate oligonucleotides, they randomized the -35 and -10 bp sequences to make a promoter library. <cite>#Saurer2010</cite> The result is a library of promoters that are highly predictable and minimize effects of from the surrounding genome. | ||
====GPD Yeast Promoter==== | ====GPD Yeast Promoter==== | ||
| Line 51: | Line 51: | ||
=====Rho-Independent===== | =====Rho-Independent===== | ||
These terminators are composed of ~11 A, and ~12 T rich nt sequences as well as a two-fold symmetric DNA sequence rich in G+C. When transcribed by RNA, these sequences lead to the formation hairpin loop rich in G-C base pairs. The formation of the RNA G-C rich stem loop causes a pause in the RNA Polymerase. This pause, followed by the transcription of the poly A tail into a run of U's causes a mechanical stress and the unwinding of the RNA-DNA complex, causing the dissociation of the RNA transcript from RNA polymerase.<cite>#Wilson1995</cite> Rho-independent terminators may have a stabilization effect on the gene they succeed. | These terminators are composed of ~11 A, and ~12 T rich nt sequences as well as a two-fold symmetric DNA sequence rich in G+C. When transcribed by RNA, these sequences lead to the formation hairpin loop rich in G-C base pairs. The formation of the RNA G-C rich stem loop causes a pause in the RNA Polymerase. This pause, followed by the transcription of the poly A tail into a run of U's causes a mechanical stress and the unwinding of the RNA-DNA complex, causing the dissociation of the RNA transcript from RNA polymerase. This type of termination is referred to as intrinsic termination.<cite>#Wilson1995</cite> Rho-independent terminators may have a stabilization effect on the gene they succeed. | ||
===='''Eukaryotic'''==== | ===='''Eukaryotic'''==== | ||
| Line 94: | Line 94: | ||
#kang2004 pmid=15615852 | #kang2004 pmid=15615852 | ||
//rrnB operon t1 terminator mechanism | //rrnB operon t1 terminator mechanism | ||
#ishihama pmid=2259628 | |||
//Strength determination of promoters | |||
Latest revision as of 13:38, 4 March 2012
Transcription
The first step toward gene expression is the transcription of a DNA template into a complementary RNA strand. This process is done by RNA polymerase, which reads the DNA template and produces an antiparallel RNA copy. As in DNA replication, the complementary strand is produced 5'->3'If the DNA template encodes for a gene, this RNA transcript will be refined into mRNA, which is further translated into a functional protein. The RNA transcript may also go on to make ribosomal RNA (rRNA), transfer RNA (tRNA), or many other RNA products. The entire process can be broken into three major steps: initiation, elongation, and termination.
Initiation
Initiation of transcription occurs differently in eukaryotes and prokaryotes. In eukaryotes, the transcription initiation complex must be formed. This includes, the core promoter, transcription factors, RNA polymerase, and activators/repressors. In bacteria, RNA polymerase and sigma factors are needed, as well, it may be necessary to have activators/repressors based on the promoter being used. For bacteria, the RNA polymerase will bind tightly to the promoter to form an open promoter complex, then must choose the transcription start site and escape from the promoter. It is necessary to balance the strength of promoter binding the ability to escape so elongation can happen. RNAP may undergo abortive initiation in which it will form many short 9-10 bp segments until it clears the promoter and begins elongation.
Promoters
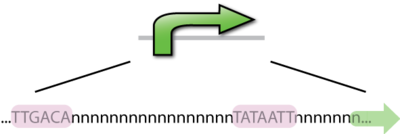
A DNA sequence that recruits transcriptional machinery and lead to transcription of downstream DNA. In E. coli the -10bp and -35bp are locations of the most well conserved DNA sequences in E. coli promoters. There are on average 17 bp between the two sequences and 7 bp between the -10bp location and the transcription start site. Consensus sequences are the nucleotide sequences that share a common function, which is binding to RNAP in the case of promoters. Promoters that most closely resemble the consensus sequence will be the strongest promoters, just as those that differ from the consensus sequence will be weaker promoters. Interestingly, there has not been a promoter found in E. coli that is of the consensus sequence, it would likely bind so strongly that elongation would not occur.
Constitutive
An unregulated promoter that allows for continual transcription of its associated genes. These promoters do not rely on input and depend only the level of free RNA polymerase. Since the holoenzyme is needed, it can also be said that these rely on the level of sigma factors, but this won't be the limiting factor.
Positive, Negative, and Multi-regulated promoters
These promoters depend on the level of transcription factors that are not sigma factors. In positively regulated, as the concentration of activator increase, the rate of transcription also increases. If an activator protein relies on the binding of an exogenous molecule to activate it, then the promoter may be referred to as inducible. For negative promoters, increased levels of a repressor will lower the activity of these promoters. If a repressor that inactivates the promoter is always present and an exogenous molecule is added that binds the repressor and deactivates it, then promoter may be referred to as inducible. Multi-regulated promoters are either positively or negatively regulated by multiple transcription factors. These are most useful when a promoter that relies on multiple environmental factors to function is desired.
Prokaryotic Sigma Factors
The E.coli RNA polymerase consist s of 5 subunits:2α, β, β', ω. The sigma factor is the 6th subunit, it is needed in forming the RNAP holoenzyme complex which is necessary in promoter binding. The sigma factor helps to recognize the -10 and -35 bp segments of the promoter. The most common sigma factor used in E. coli is the σ70 subunit. This is the housekeeping sigma factor and is used during transcription of most genes. It recognizes the consensus sequence: TTGACA__(17)__TATAAT. [2]There are an additional 6 sigma factors, active in different situations. Such as σ32, which is the heat shock sigma factor. The B. subtilis housekeeping sigma factor is σA, similar to σ70 in E. coli.
Determining Strength
The strength of the different promoters is determined by the relative frequency of transcription initiation. This is mainly affected by the affinity of the promoter sequence for RNA polymerase. [3]Quantitatively measuring this can be done by monitoring protein synthesis rate of a protein such as GFP. Promoters that differ significantly from the consensus sequence will be weaker than those that resemble the consensus sequence due to binding affinity.[4]
Promoter Examples
E. coli Promoter: LacUV5

LacUV5 is a constitutive promoter mutated from of the lac promoter found in E. coli. The lac promoter is considered weak, it varies from the consensus sequence by 3 bases. On the other hand, the lacUV5 mutated promoter varies from the consensus sequence by only 1 base and is much stronger than the lac promoter. Even though lacUV5 is considered strong relative to other E. coli promoters, these are still weak when compared to T7 promoters.
Strong Promoter: Bacteriophage T7 Promoter
The T7 promoter is derived from bacteriophage T7. The T7 RNA polymerase has a very high affinity for its own promoters which do not occur naturally in E. coli, it also very efficient resulting in elongation that is five-fold faster than E. coli RNAP. In the experiment done by moffatt et al. the gene transcribing T7 RNAP was introduced under the control of the lacUV5 promoter. They showed that the T7 RNAP will transcribe almost any gene connected to a T7 promoter introduced into the E. coli genome. It was found that even with a small amount of T7 RNAP, the mRNA transcripts were saturating the translational machinery of E. coli. A target protein could accumulate up to 50% of the total cellular protein in ~3 hours.[6]
Constitutive Bacterial Promoter Design

Sauer et al. generate various promoters spanning two orders in magnitude of strength. The promoters were based on the E. coli rrnB P1, a strong σ70-dependent promoter with near consensus −10 (TATAAT) and −35 (TTtACg) elements. The region from position -105 to +55 were insulated by nucleotide sequences that did not bind proteins, blocking all transcription factor binding sites, enhancer sites, as well as defining a specific 5' mRNA start site. Using proD, their first generation insulated promoter, the production of the GFP reporter gene was driven. Measuring strength of this promoter relative to another, the GFP synthesis rate was monitored. They found that the insulated proD promoter performed significantly better than a minimal promoter. Through the use of degenerate oligonucleotides, they randomized the -35 and -10 bp sequences to make a promoter library. [7] The result is a library of promoters that are highly predictable and minimize effects of from the surrounding genome.
GPD Yeast Promoter
A constitutive yeast promoter is the GPD promoter. Found in the registry: http://partsregistry.org/Part:BBa_K517001 The 2011 British Columbia iGEM team performed Fluorescent analysis of the GPD promoter based on a media change. As shown, there was no noticeable difference in GFP expression. [8] This same promoter is present in the addgene S. cerevisiae Advanced Gateway Destination Vectors http://www.addgene.org/yeast-gateway/. This kit allows for expression of open reading frames through either GPD or GAL1.
Termination

Terminators
Terminators consist of a G+C rich dyad symmetry sequence followed by a poly (T) tract. The iGEM registry has forward, reverse, and bidirectional terminators. The most important piece of a terminator is the G+C dyad sequence that will form a a hairpin loop in the RNA transcript. It has also been shown that the poly (T) tract is needed for termination to occur.
Prokaryotic
Rho-Dependent
In this type of termination, a protein factor called Rho destabilizes the DNA template-RNA transcript complex, causing the release of the RNA transcript. Rho-dependent terminators are not included in the iGEM registry because these terminators are not specified by sequence.
Rho-Independent
These terminators are composed of ~11 A, and ~12 T rich nt sequences as well as a two-fold symmetric DNA sequence rich in G+C. When transcribed by RNA, these sequences lead to the formation hairpin loop rich in G-C base pairs. The formation of the RNA G-C rich stem loop causes a pause in the RNA Polymerase. This pause, followed by the transcription of the poly A tail into a run of U's causes a mechanical stress and the unwinding of the RNA-DNA complex, causing the dissociation of the RNA transcript from RNA polymerase. This type of termination is referred to as intrinsic termination.[10] Rho-independent terminators may have a stabilization effect on the gene they succeed.
Eukaryotic
In yeast, termination is different for each RNA polymerase (I-III). The process for pol II, the main RNAP of eukaryotes, involves the polyadenylation at the 3' end of the RNA transcript. A set of proteins cleave off the RNA transcript and then synthesize the poly A tail, independent of the DNA template. This step is important toward refining the RNA into mRNA that will translated.
Terminator Examples
E. coli crp Terminators
The terminator of the E. coli crp gene that encodes for the cAMP receptor protein follows a rho-independent termination model. Aiba et al. showed that crp terminator assisted in stabilizing the RNA transcript of this gene. As well, it was shown that the G+C rich stem was the stabilizing factor rather than the poly A or T segments. By making variants of the crp terminator, it was shown that disruption of the G+C rich dyad almost completely eliminated terminator function. While disruption of the T tail significantly lowered terminator function, but not completely. This showed that the G+C rich dyad is the most important piece terminator with respect to function. [11]
E. coli rrnB operon t1 Terminator
This terminator stops transcription by forming an RNA hairpin followed by a U rich sequence, encoded by a class I termination signal. This is the typical termination of RNAP and is highly conserved across many species of bacteria. this specific promoter also terminates via a class II signal which affects T7 RNAP. Class II termination consists of a conserved sequence (CS), XATCTGTT (X= A, C, or T), followed by a T rich sequence. This conserve sequence is extremely specific, if any point mutation occurs, this class of termination will cease to work. [12]
ADH1 Yeast Terminator
The alcohol dehydrogenase gene terminator ADH1 is a commonly used yeast terminator in synthetic biology. It is 129bp long and is an effective terminator of transcription.
iGEM Terminators
Terminator sequences can be used to terminator forward, reverse, or bidirectional transcription. An example of a forward terminator is: http://partsregistry.org/wiki/index.php?title=Part:BBa_B0015 The double terminator, B0015, was characterized as the most efficient forward terminator (98.4%) and a functional reverse terminator (29.5% efficient). [13] There are various terminators listed, with differing efficiencies for the role needed.
References
<biblio>
- Weiss2005 pmid=16285917
//Analysis of sigma factor in initiation to elongation step
//lacUV5 and lac promoter comparison
- Wilson1995 pmid=7568019
//Formation of RNA hairpin loop and dissocation
- Moffat1985 pmid=3537305
//T7 RNAP promoter work
- Saurer2010 pmid=20843779
//Promoter library creation
//Yeast GPD promoter analysis
- kingsford2007 pmid=17313685
//Termination image
- registry http://partsregistry.org/Promoters
//Catalog of promoters
- registry2 http://partsregistry.org/Terminators
//Catalog of Terminators
- aiba1996 pmid=9150882
//E. coli crp gene terminator analysis
- kang2004 pmid=15615852
//rrnB operon t1 terminator mechanism
- ishihama pmid=2259628
//Strength determination of promoters