CH391L/S12/Unnatural Amino Acids
Unnatural Amino Acids
Introduction
Beginning with Noren et al. in 1989, various groups have expanded the genetic code in vitro and in organisms from bacteria to mammalian cells using Amber (TAG) stop codon suppression technology. Consisting of a tRNA to the TAG stop codon and an orthogonal aminoacyl-tRNA synthetase (aaRS) introduced on a plasmid, this system incorporates unnatural amino acids at TAG stop codons in vivo. In addition to the many potential applications in the study of protein and pathway engineering, these systems provide a unique opportunity to study the evolution of organisms forced to utilize a 21st amino acid. In this project, we will observe the long- term evolution of two E. coli strains, each separately carrying seven different UAA suppression systems. Strains will be passaged daily in a minimal, defined liquid media supplemented with the UAA to allow for selection. Each will also undergo single-cell bottlenecking daily on minimal agar media + UAA to observe genetic drift. Using plasmid and whole- genome sequencing, the adaptation and long-term evolution of both the engineered system and the genome will be observed. Anticipated results include UAA system adaptation and optimization, rapid stop codon mutation, dynamic genomic evolution, and unnatural amino acid addiction .
Chassis Organisms
E. coli Strains
Cloning
Protein Expression
Orthogonality
Genome Reduction
Systematic Genome Reduction
One natural approach to engineering strains with a reduced genome is to systematically identify and delete regions of the genome not necessary for host cell survival. Posfai et al. created the MDS strains (multiple deletion strains) by aligning the genomes of multiple genomes of E. coli, identifying regions which were absent in multiple strains, and deleting them via Lambda Red recombination. All IS elements were removed as well, lowering the mutation rate and increasing the stability of genetic constructs introduced into the cell. The strain had comparable growth rate compared to wild type.[1][2]
Ara et al. constructed a minimal version of the B. subtilis genome in 2007. [3] This strain had slightly decreased growth rate compared to wild-type, but displayed normal morphology and similar protein production capabilities.
Selection for Reduced Genome
Long-term evolution of strains under the correct conditions could select for a genome of minimal size. Such conditions may include growth in rich media lacking sugars to favor the loss of biosynthetic pathways or sugar metabolism operons, growth in structured environments which favor a smaller cell, or growth under other conditions which favor the loss of unnecessary genes.

Minimal Genome Synthesis
Another approach is the synthesis of a minimal, designed genome from scratch using DNA synthesis technology and the transformation of this genome into cells to create a viable, novel, synthetic organism. This approach can be viewed as forward engineering of a novel organism, but would likely be informed by studies which determine the minimal set of genes necessary for a living organism.
Mycoplasma mycoides Synthesis
Gibson et al synthesized the first artificial cell by generating the Mycoplasma mycoides genome from digitized genome information and transforming it into Mycoplasma capricolum cells devoid of genomic information. These cells were capable of continuous self-replication and were identified by "watermarks" inserted in the genome. This technology could be utilized in the future to create cells with novel and useful properties from scratch.[4][5]
Yeast as a Model Organism
The following table summarizes some of the advantages and disadvantages of using yeast as a model organism for synthetic biology. [6]
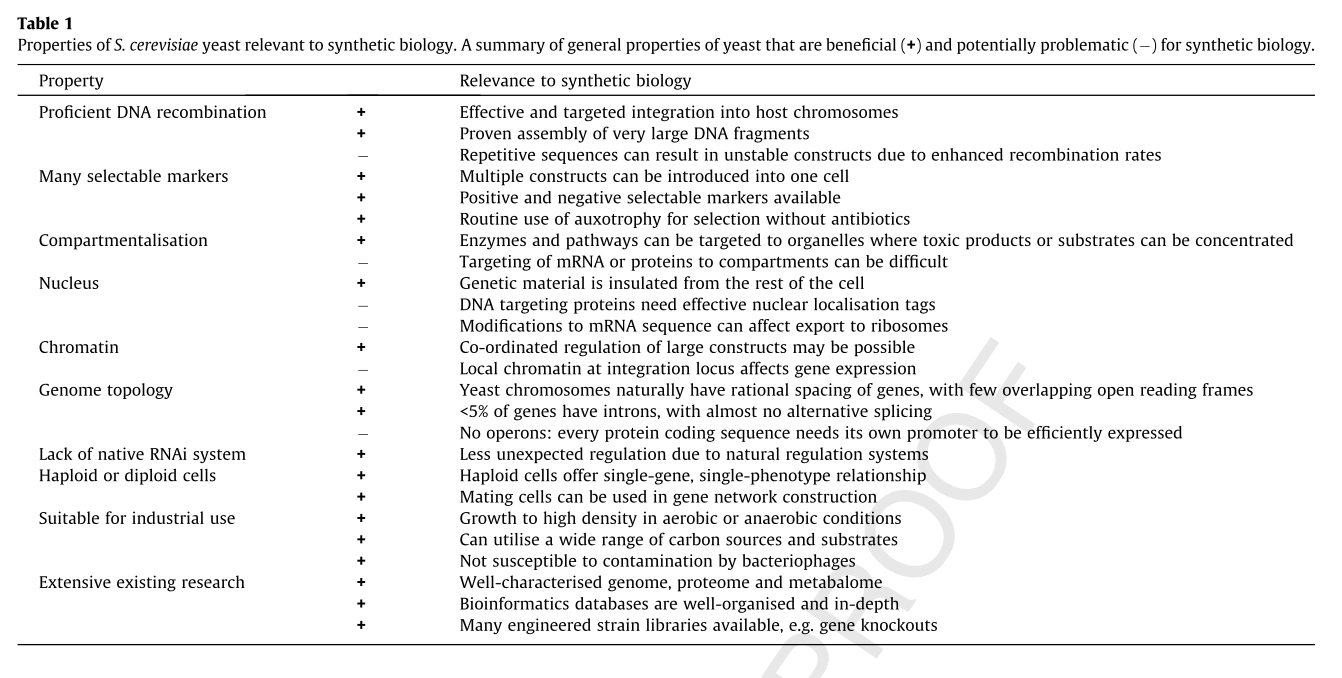
References
- Kolisnychenko V, Plunkett G 3rd, Herring CD, Fehér T, Pósfai J, Blattner FR, and Pósfai G. Engineering a reduced Escherichia coli genome. Genome Res. 2002 Apr;12(4):640-7. DOI:10.1101/gr.217202 |
- Pósfai G, Plunkett G 3rd, Fehér T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, Burland V, Harcum SW, and Blattner FR. Emergent properties of reduced-genome Escherichia coli. Science. 2006 May 19;312(5776):1044-6. DOI:10.1126/science.1126439 |
- Ara K, Ozaki K, Nakamura K, Yamane K, Sekiguchi J, and Ogasawara N. Bacillus minimum genome factory: effective utilization of microbial genome information. Biotechnol Appl Biochem. 2007 Mar;46(Pt 3):169-78. DOI:10.1042/BA20060111 |
- Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, Zaveri J, Stockwell TB, Brownley A, Thomas DW, Algire MA, Merryman C, Young L, Noskov VN, Glass JI, Venter JC, Hutchison CA 3rd, and Smith HO. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008 Feb 29;319(5867):1215-20. DOI:10.1126/science.1151721 |
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA 3rd, Smith HO, and Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010 Jul 2;329(5987):52-6. DOI:10.1126/science.1190719 |
- Blount BA, Weenink T, and Ellis T. Construction of synthetic regulatory networks in yeast. FEBS Lett. 2012 Jul 16;586(15):2112-21. DOI:10.1016/j.febslet.2012.01.053 |
- Metzgar D, Bacher JM, Pezo V, Reader J, Döring V, Schimmel P, Marlière P, and de Crécy-Lagard V. Acinetobacter sp. ADP1: an ideal model organism for genetic analysis and genome engineering. Nucleic Acids Res. 2004;32(19):5780-90. DOI:10.1093/nar/gkh881 |
- An W and Chin JW. Synthesis of orthogonal transcription-translation networks. Proc Natl Acad Sci U S A. 2009 May 26;106(21):8477-82. DOI:10.1073/pnas.0900267106 |