Chimeras: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
== Current Trends in Biomedical Research == | == Current Trends in Biomedical Research == | ||
[[Image:organtransplant.jpg|thumb|right|460px| '''Figure 1.''' Organ Transplant. [2]]] | [[Image:organtransplant.jpg|thumb|right|460px| '''Figure 1.''' Organ Transplant. [2]]] | ||
Every day, nearly 200 people die awaiting an organ transplant because of the lack of suitable and viable organs available [1]. It is a long standing issue in biomedical and tissue engineering research where the number of available organ donors isn't nearly enough to compensate for the number of organs needed for transplants. Scientists are looking towards alternative methods, mainly involving stem cells | Every day, nearly 200 people die awaiting an organ transplant because of the lack of suitable and viable organs available [1]. It is a long standing issue in biomedical and tissue engineering research where the number of available organ donors isn't nearly enough to compensate for the number of organs needed for transplants. Scientists are looking towards alternative methods, mainly involving stem cells, | ||
== History of Organ Transplants == | |||
[[Image:Spinalcord.jpg|thumb|right|300px| '''Figure 3.''' Anatomy of the spinal cord. [10]]] | [[Image:Spinalcord.jpg|thumb|right|300px| '''Figure 3.''' Anatomy of the spinal cord. [10]]] | ||
Revision as of 16:58, 22 April 2017
Current Trends in Biomedical Research

Every day, nearly 200 people die awaiting an organ transplant because of the lack of suitable and viable organs available [1]. It is a long standing issue in biomedical and tissue engineering research where the number of available organ donors isn't nearly enough to compensate for the number of organs needed for transplants. Scientists are looking towards alternative methods, mainly involving stem cells,
History of Organ Transplants

The Spinal Cord
The spinal cord is the main channel between brain and body where sensory and motor information travel [11]. This information travels along specifically organized nerve fibers which extend to all parts of the body, delivering sensory information to the CNS and motor information to the periphery to innervate smooth and skeletal muscles that control involuntary and voluntary functions [11]. It helps facilitate the control of not only motor functions of the extremities but also daily functions of organs in the body. The spinal cord runs along the back encased and protected by the vertebrae (spinal column) [1] and is the location where all the major nerves in the body meet. The spinal column is a collection of 33 bones, 7 make up the cervical (neck) region, 12 constitute the thoracic (chest) region, 5 make up the lumbar (lower back) region, 5 in the sacral region and 4 in the coccygeal (tailbone) region [12]. 31 nerve pairs in total are responsible for sending information throughout the body, and their distribution among the five vertebrae regions is illustrated in Figure 3 [10]. Injury along the spinal cord is normally extremely detrimental and usually results in paralysis.
Spinal Cord Injuries
Unlike other major parts of the human body, the spinal cord lacks the ability to heal itself following injury. Spinal cord injuries (SCIs) are characterized by a loss of nervous tissue, and accordingly, a loss of motor and sensory functions. SCIs can be caused from numerous things such as trauma, loss of normal blood supply, or compression resulting from bone fractures, hematoma, tumors or a herniated disk [13]. SCIs are most common among white males and victims of trauma, often as a result of automobile accidents (40%), direct wounds from violence (gunshot wounds or stabbings)(16%), high falls and contact in sports [13]. SCIs can also be categorized as being complete or incomplete. A complete SCI is characterized by the total loss of function below the level of injury along the spinal column, which leads to a type of paralysis. For example, an injury in one of the lower thoracic nerves (T6-T12) will usually cause a person to become a paraplegic, meaning loss of function below the waist and in both legs, shown in Figure 4 [14]. An incomplete SCI will allow a person to maintain some function and mobilization below the level of injury [13]. In the United States alone, there are near 12,000 new cases of SCIs annually, and at any time there are around 200,000 people living with a SCI [13].

Pathophysiology of Spinal Cord Injuries
A great understanding of the underlying cellular damage mechanisms following a spinal cord injury is crucial to creating an effective and preventative cure. The injury can be categorized into two phases, the primary mechanical phase and the secondary biochemical and vasculature injury cascade phase [20]. As soon as the injury occurs, the normal architecture of the spinal cord is ruptured at a certain location, followed by damage and cell death of neurons and glial cells and demyelination of axons which leads to discontinuity between sensory and motor signals and functions, (e.g. paralysis) [20]. The level of injury along the column and the degree of damage is the crucial first step in determining areas of completely lost functions. Ischema is usually followed, along with accumulation of neurotransmitter, which causes axonal conduction block, blocking sensory information signaling. Ischema and acute inflammatory response lead to secondary injury, where inflammatory cells home to the site of injury, release pro-inflammatory cytokines and clear cellular debris, while also killing normal tissue and inducing the formation of glial scars which prevent the growth of neurons [20].
Past and Present Treatments for SCIs
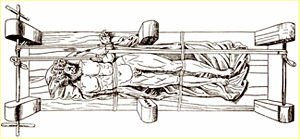
In ancient times, SCIs were treated by rest, immobilization, and traction. In Greece, Hippocrates invented an extension bench, like the one seen in Figure 5 [15], which provides a person suffering from an SCI or a spine deformity with immobilization and traction using a stiff board and ropes [15]. Spinal manipulations like this characterized early treatments for SCIs. With the discovery of X-rays in 1920, SCI injuries were easier to locate and diagnosis and treatment was more accurate. During the middle of the twentieth century, a standardized method for treating SCIs was established that involved repositioning of the spine so that it remained in a certain position and rehabilitation with exercise [13].
Today, SCIs are most commonly treated with immobilization, surgery and rehabilitation. Many of the current treatments focus on rehabilitation, which helps SCI patients develop ways to become accustomed to movement in their daily life with their injuries. Within hours of the initial injury, high doses of steroids are normally administered to reduce inflammation and swelling at the site of injury, which would otherwise compress the spinal cord and delay the recovery process. The patient is immobilized to prevent further damage to the spine, and surgery is normally performed to accomplish two main goals: (1) relieve any pressure on the spine from compression and (2) stabilization of the spine with plates and other hardware, allowing the bones time and space to heal correctly [13]. Following surgery, rehabilitation is carried out in forms of physical and occupational therapy. Currently, there is no cure for SCIs.
Stem Cell-Based Therapies for Spinal Cord Injury
Cell based therapies have been gaining the research spotlight to finding a better treatment or possibly even a cure for SCIs. Due to the pluripotent and self-renewing nature of stem cells, favorable experimental results in vitro have found that stem cells can be directed to differentiate into cells of the central nervous system, including glia or neurons, as shown in Figure 6 [16]. These cells can be used as replacements for neural cells lost following SCIs. Stem cells implanted into the site of injury can promote axon regeneration and neuroprotection upon secretion of certain growth factors, while also aiding in the spinal cord repair process [16].

Using ESCs to Treat SCIs in Rodents
Su Liu (Washington University School of Medicine and the Center for the Study of Nervous System Injury) published one of the first papers that showed differentiation of ESCs into oligodendrocytes to help treat SCIs [16]. Oligodendrocytes are a subset of glial cells, are a main component of the central nervous system and are specifically involved with myelinating axons of cells. The myelin sheath surrounding the axon of some nerve cells helps propagate action potential down the cell, allowing signals to reach other cells. This signaling process is essential to the central nervous system and results in body functions (eg. muscle movement) [16]. Following a spinal cord injury, demyelination occurs and transmittance of these signals is disrupted, resulting in cell death and paralysis. Oligodendrocytes can help fix these damaged cells by repairing axons via re-myelination, which increases the success of action potential traveling, promotes signal propagation, and potentially restores motor function. In the study, ESC-derived cells were shown to myelinate axons in vitro and in vivo following transplantation into the spinal cord of adult rats three days after chemical demyelination. This study also verified that ESCs could survive, differentiate and produce myelin in the myelin deficient spinal cord environment [16]. Since the late 1990s, many animal studies have been published that confirm the potential of stem cells to repair and regenerate cells of the CNS. iPSCs are under recent investigation as a potential therapeutic treatment because of their similarity to ESCs and their ability to mimic many of the functions of ESCs. iPSC experiments have yet to expand from in vitro and in vivo models. Early animal studies have shown iPSCs have similar benefits to ESCs, with the added benefit of not having the ethical concerns associated with using ESCs.
Using ESCs to Treat SCIs in Patients
In 2010, Geron Corporation started the first clinical trial using hESC-based therapy to treat SCIs based on Keirstead’s work [17]. This study was titled: Phase 1 Safety Study of GRNOPC1 in Patients With Neurologically Complete, Subacute, Spinal Cord Injury [18]. GRNOPC1 is the population of cells that are hESC-derived oligodendrocyte progenitor cells (OPCs). Four patients were given one injection of 2 million cells. Following one year after transplantation, no teratomas were found, small, non-harmful cysts were found at injection sites, no toxicity or cell migration outside the central nervous system, no stimulated pain, and no major susceptibility to immune attack [18]. Although the trial ended following Phase I, Geron will continue to monitor the health and well-being of the patients for the next fifteen years. Interestingly, Geron's IND application for the trial was close to 28,000 pages in length, one of the longest ever submitted to the FDA [19]. Currently, there are twelve open clinical studies using stem cells to treat spinal cord injuries, eight out of twelve of them using mesenchymal stem cells and none using ESCs. The majority of this subset is looking at transplantation of autologous MSCs for treatment in complete spinal cord injuries. Desired participant ages range from 12 months to 65 years.
MSCs for Spinal Cord Injuries
MSCs have been found to secrete neurotrophic molecules, promote neural generation and rescue impaired neural function following transplantation in models of SCI [20]. Neurotrophic molecules are responsible for the growth and proliferation of mature and developing neurons. MSCs are especially intriguing because of their immunomodulatory mechanisms at sites of injury and their ability to forgo graft rejection in humans. They can carry out these functions after being directed under certain conditions to differentiate into cells of the neuronal and glial lineages. Transplanted MSCs have also been found to exert other therapeutic effects such as promoting angiogenesis and anti-inflammatory actions which can aid in neural regeneration and reduction of glial scar formation [20]. Recently, in March 2016 in a Medical Center in Seoul, a phase III clinical trial was underway for the treatment of spinal cord injury using autologous MSCs [21]. Phase II data showed 60% improvement in neurological function of the upper extremities in patients suffering from chronic SCI, 3 of whom showed increased activity of daily life activities. Following MSC transplantation in Phase III, two of the sixteen patients involved showed increased motor improvements of upper extremities, and no changes were observed in the remaining patients, which is not enough to fulfill the effectiveness criteria for passing a drug or treatment. From phase II to phase III study, injections were diminished from three to one in order to comply with the regulation of a single application for treatment for new drugs, which was cause for the decreased efficacy in phase III of the treatment [21]. MSCs have been the majority cell type studied in clinical and preclinical trials for the treatment of SCIs.
Stem Cell Institute: Panama [2]
Currently, the Stem Cell Institute in Panama is offering autologous MSC and umbilical cord derived allogeneic MSC transplants for treating patients suffering from SCIs. Treatment involves a total of 16 injections over the course of four weeks, administering both sets of stem cells in a set order both intravenously and intrathecally (into spinal fluid). Previous patients frequently share their success stories and promote the program at talks and webcasts, where they express how the regain of some function in their arms or legs has changed their life for the better [22].
Christopher and Dana Reeve Foundation [3]
Christopher Reeve, the original Superman and a leading actor, director and activist in his time, became a full tetraplegia following an equestrian accident in 1995. After his injury, he became an inspiration and motivation for neuroscientists to go into power drive in researching treatments for one of the most misunderstood and troubling diseases of the central nervous system [23]. The foundation, initially established by himself and his wife, seen in Figure 7 [23], is dedicated to finding cures for spinal cord injuries by funding groundbreaking research and providing grants, information and advocacy to improve the lives of patients with SCIs.

Advantages and Disadvantages of Cell-Based Therapies for SCIs
ESCs can proliferate in vitro to produce large amounts of neuron and glial precursor cells. It is much more difficult to obtain a large amount of these cell types from adult stem cells (ASCs), because some ASCs are hard to locate and remove from the body, since most have differentiated into a specialized cell [20]. There are still many ethical concerns surrounding the use of ESCs however, because there are questions to at which point a fetus becomes a human. ASCs (like MSCs) are genetically identical to the recipient (autologous stem cell transplant) and therefore the risk for transplant rejection is diminished. ESCs, however, are genetically non-identical from donor, and long-term immunosuppressants may be necessary to decrease the risk of opportunistic infections [20]. There are also still concerns about what happens to the cells once they are in the spinal cord environment, for example, if they differentiate into other cells or migrate to other areas of the body. MSCs differentiate into a specific cell lineage in the body, so there are still questions as to how well MSCs can function as nerve cells [20]. They may also not last as long as ESCs. There is also concern from past evidence of ESCs forming teratomas inside the body. iPSCs are promising because they can be immunologically compatible with the host (autologous), however the reprogramming process takes several months and the procedure is still not perfect (risk of unpredicted mutations and clone to clone variation) [20].
Conclusions and Future Work
Stem cells show great promise for treatment of spinal cord injuries, one of the most debilitating and incurable conditions to date. However many questions still remain to be answered; what stem cell is most appropriate for treatment, or combination thereof, and what exactly happens to the stem or progenitor cells once inside the body. Clinical trials in the United States have yet to see later phases, so long term efficacy for this type of treatment has yet to be realized. An effective treatment strategy is also crucial for the continued advancement of stem cell therapies for treatment of SCIs. Due to the complex pathophysiology following a SCI, including primary and secondary mechanisms, it is difficult to develop therapies for success in clinical trials. The current clinical trials are aimed at reversing only the damaging effects seen during the secondary stage of SCI. Even with clinical trials in play, numbers of participants are in the single and low double digits, and progress towards any results are very, very slow. In the future, a hope for a treatment for SCIs is dependent on more stringent and focused preclinical and clinical trials, and further understanding of the role stem cells play in the SCI environment.
References
[1] Howard, D., Buttery, L. D., Shakesheff, K. M., Roberts, S. J. (2008). Tissue engineering: strategies, stem cells and scaffolds. Journal of Anatomy, 213(1), 66–72. [4]
[2] BioCat GmbH. Stem Cell Differentiation Reporter System. [5] (accessed Feb 13, 2017).
[3] Loh, Y.-H., Wu, Q., et al. (2006). The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nature Genetics, 38(4), 431–440. [6]
[4] Nakamura, M., Okano, H. (2012). Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Research, 23(1), 70–80. [7]
[5] Sheng, G. (2015). The developmental basis of mesenchymal stem/stromal cells (MSCs). BMC Developmental Biology 15(1). [8]
[6] The San Antonio Orthopaedic Group, LLP. Orthopedic Stem Cell Therapy. [9] (accessed Feb 13, 2017).
[7] Murnaghan, I. History of Stem Cell Research. [10] (accessed Feb 11, 2017).
[8] David, S., Aguayo, A. J. (1985). Axonal regeneration after crush injury of rat central nervous system fibres innervating peripheral nerve grafts. Journal of Neurocytology, 14(1), 1–12. [11]
[9] Thomson, J. A. (1998). Embryonic Stem Cell Lines Derived from Human Blastocysts. Science, 282(5391), 1145–1147.
[10] McGovern Medical School at UT Health. Chapter 3: Anatomy of the Spinal Cord. [12] (accessed Feb 13, 2017).
[11] Jr, F. M. M., Bracken, M. B., Creasey, G., et al. (1997). International Standards for Neurological and Functional Classification of Spinal Cord Injury. Spinal Cord, 35(5), 266–274. [13]
[12] Sargon, M. F. (2009). The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas by Charles Watson, George Paxinos and Gülgün Kayalıoğlu. Anatomy, 3, 73–75. [14]
[13] Eck, J. C., Marks, J. W. Spinal Cord Injury: Levels, Treatment, Symptoms, Recovery. [15] (accessed Feb 11, 2017).
[14] Regeneration Center of Thailand. Treat Spinal Cord Injuries With Neuro MSC+ Stem Cells. [16] (accessed Feb 11, 2017).
[15] Vasiliadis, E. S., Grivas, T. B., Kaspiris, A. (2009). Historical overview of spinal deformities in ancient Greece. Scoliosis, 4(1). [17]
[16] Liu S., et al. (2000). Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. PNAS (97): 6126-31. [18]
[17] Keirstead, H.S., et al. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of Neuroscience (19): 4694-4705. [19]
[18] Geron Press Release. Geron Presents Clinical Data Update from GRNOPC1 Spinal Cord Injury Trial. [20] (accused Feb 13, 2017].
[19] The New York Times. Stem Cell Trial Wins Approval of F.D.A. [21] (accessed Feb 13, 2017).
[20] Goel, A. (2016). Stem cell therapy in spinal cord injury: Hollow promise or promising science? Journal of Craniovertebral Junction and Spine, 7(2), 121. [22]
[21] Oh, S. K., Choi, K. H., Yoo, J. Y., et al. (2016). A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery, 78(3), 436–447. [23]
[22] Stem Cell Institute Panama. Spinal Cord Injury. [24] (accessed Feb 13, 2017).
[23] Christopher and Dana Reeve Foundation. History of the Reeve Foundation. [25] (accessed Feb 13, 2017).
