Dave Gray's Build-A-Gene Class Notes - Session 4: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
Lisa explained the rationale for the restriction enzymes that were chosen and some options for making this all work. | Lisa explained the rationale for the restriction enzymes that were chosen and some options for making this all work. | ||
[[Image:Session4.png]] | [[Image:Session4.png|frame|border|center|middle|||alt=The restriction enzymes|This shows how the restriction enzymes were selected for cutting the ends of our vector components. EcoRI and PstI were able to cut ends of two different components so that those ends could then join. These ends could later be cut again for further splicing by the same enzymes. For the third connection, the two ends were cut by separate restriction enzymes - SpeI and XbaI. Although two different enzymes were used, the ends were compatible. Once joined, however, the joint cannot be cut by the same restriction enzymes because the resulting nucleotide sequence doesn't satisfy the requirements of either enzyme. This is referred to as a "scar".]] | ||
Some of the "tweaking" can be done in a DNA sequence to facilitate the use of restriction enzymes. For example, when planning this, you would have to ensure that there were no other occurrences of the nucleotide sequence that a restriction enzyme matches to such that it would cut your component where you don't want. The way nucleotides are matched to amino acids in assembling proteins offers one solution. In coding for amino acids, a set of three nucleotides is always selects a specific amino acid. However, many amino acids can be selected by more than one nucleotide sequence. So one triplet of nucleotides could be replaced with another that codes for the same amino acid without breaking the function of the genome. | |||
This also explains one possible reason why we used three components. Logically, we could have combined the vector and promoter into a single strand. However, if PstI could cut the promoter at a location we don't want or SpeI would cut the vector at a location we didn't want, that would be a problem. However, by handling them as separate strands, that possibility is avoided. Before combining components, we heat them separately to 80°C. This deactivates the enzymes so that the components can be safely mixed. | |||
We talked a bit about how restriction enzymes work. One point was that in the double helix of DNA, there is also a second double helix of "grooves" in the space between the backbones of the two strands. One side is larger and the other is smaller. These are referred to as "major" and "minor" grooves. Restriction enzymes tend to bind at points in the major groove. (It allows more space for them to access the nucleotides.) | |||
Lisa explained that a vector like the one we constructed could also be used in yeast or mammalian cells. For it to be used there, we would have to add an appropriate origin of replication (origin or ori) for one or both of these. | |||
It turns out that only a very small number of bacteria take up our vector. (e.g. 1/1,000,000) For that reason, we need a LOT of bacteria and a LOT of vectors. To weed out those that have from those that have not, our vector includes a selectable marker. We used the Chlor<sup>r</sup> gene. It allows the bacteria to live in the presence of the antibiotic chloramphenicol. Then, by including that antibiotic in the growth material in the petri dishes, we ensure that only bacteria with our vector will survive and grow. (Many of these will have flaws in the vector and so will not fluoresce green. However, they will be far more concentrated. | |||
A tip from the class - always label the bottom of the petri dish, not the top. That way, if you drop them and the lid goes rolling off, you haven't lost your label. | |||
'''partsregistry.org''' | |||
Lisa mentioned the standard registry of biological parts at partsregistry.org (now http://parts.igem.org/Main_Page). This allows for labs to share reusable parts. (Not all work as advertised.) She plans to submit our EmGFP gene to the registry after our class is complete. | |||
'''IGEM - International Genetically Engineered Competition''' | |||
We discussed the IGEM competition, a competition for genetically engineered projects. Two commercial spinoffs from IGEM are Ginko Bioworks and BioFab. | |||
[[Dave Gray's Build-A-Gene Class Notes - Session 3 | Previous]] | [[Dave Gray's Build-A-Gene Class Notes | Main Page]] | [[Dave Gray's Build-A-Gene Class Notes Glossary | Glossary]] | [[Dave Gray's Build-A-Gene Class Notes - Session 5 | Next]] | [[Dave Gray's Build-A-Gene Class Notes - Session 3 | Previous]] | [[Dave Gray's Build-A-Gene Class Notes | Main Page]] | [[Dave Gray's Build-A-Gene Class Notes Glossary | Glossary]] | [[Dave Gray's Build-A-Gene Class Notes - Session 5 | Next]] | ||
Revision as of 20:46, 21 August 2013
In session 4 we did ligation of our vectors and transformation of E. coli. We also had a discussion of current work in molecular biology.
Ligation
At the end of the previous session, we added restriction enzymes to our vector, promoter and emGFP gene and left them to work so that the restriction enzymes could snip the ends of each of these, leaving "sticky ends" that would allow the three pieces to be joined into a continuous loop, initially by hydrogen bonds due to the attraction of the nucleotides, then with covalent bonds by a process called ligation that "zips up" the DNA backbone at the junction points.
To perform this, we combined all three of our components with T4 ligase + buffer solution and incubated, first for 30 minutes at 16°C, then for 20 minutes at 80°C.
Lisa explained the rationale for the restriction enzymes that were chosen and some options for making this all work.
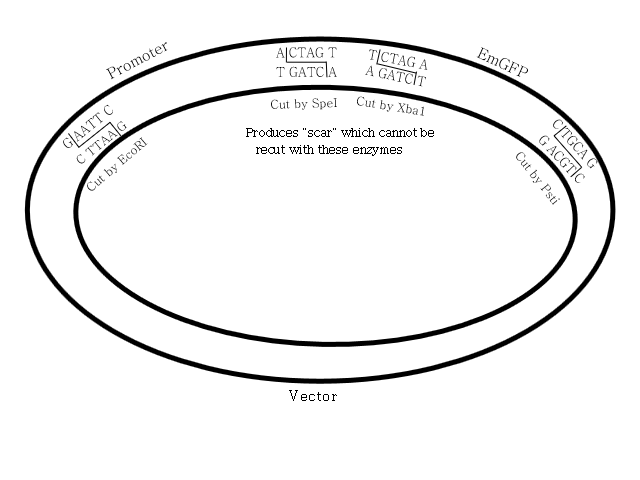
Some of the "tweaking" can be done in a DNA sequence to facilitate the use of restriction enzymes. For example, when planning this, you would have to ensure that there were no other occurrences of the nucleotide sequence that a restriction enzyme matches to such that it would cut your component where you don't want. The way nucleotides are matched to amino acids in assembling proteins offers one solution. In coding for amino acids, a set of three nucleotides is always selects a specific amino acid. However, many amino acids can be selected by more than one nucleotide sequence. So one triplet of nucleotides could be replaced with another that codes for the same amino acid without breaking the function of the genome.
This also explains one possible reason why we used three components. Logically, we could have combined the vector and promoter into a single strand. However, if PstI could cut the promoter at a location we don't want or SpeI would cut the vector at a location we didn't want, that would be a problem. However, by handling them as separate strands, that possibility is avoided. Before combining components, we heat them separately to 80°C. This deactivates the enzymes so that the components can be safely mixed.
We talked a bit about how restriction enzymes work. One point was that in the double helix of DNA, there is also a second double helix of "grooves" in the space between the backbones of the two strands. One side is larger and the other is smaller. These are referred to as "major" and "minor" grooves. Restriction enzymes tend to bind at points in the major groove. (It allows more space for them to access the nucleotides.)
Lisa explained that a vector like the one we constructed could also be used in yeast or mammalian cells. For it to be used there, we would have to add an appropriate origin of replication (origin or ori) for one or both of these.
It turns out that only a very small number of bacteria take up our vector. (e.g. 1/1,000,000) For that reason, we need a LOT of bacteria and a LOT of vectors. To weed out those that have from those that have not, our vector includes a selectable marker. We used the Chlorr gene. It allows the bacteria to live in the presence of the antibiotic chloramphenicol. Then, by including that antibiotic in the growth material in the petri dishes, we ensure that only bacteria with our vector will survive and grow. (Many of these will have flaws in the vector and so will not fluoresce green. However, they will be far more concentrated.
A tip from the class - always label the bottom of the petri dish, not the top. That way, if you drop them and the lid goes rolling off, you haven't lost your label.
partsregistry.org
Lisa mentioned the standard registry of biological parts at partsregistry.org (now http://parts.igem.org/Main_Page). This allows for labs to share reusable parts. (Not all work as advertised.) She plans to submit our EmGFP gene to the registry after our class is complete.
IGEM - International Genetically Engineered Competition
We discussed the IGEM competition, a competition for genetically engineered projects. Two commercial spinoffs from IGEM are Ginko Bioworks and BioFab.