Drug-Eluting NanoPolymers by Gladys Saruchera
Motivation

There is an increasing interest in studying the field of drug delivery. This is because most medications, if not delivered to the site of disease, will cause damage to healthy cells. For example, in the treatment of cancer, most medications used treat tumors by interrupting the cell replication process [1]. If not targeted, healthy cell replication needed for growth and tissue regeneration will be disrupted, leading to side effects such as weight and hair loss associated with chemotherapy. It is therefore necessary to find ways to deliver drugs to diseased cells, with minimal exposure of healthy cells.
Another permanent challenge in administering most drugs is the issue of biocompatibility. The immune system recognizes most drug molecules as foreign objects, thus eliminates them from the bloodstream before they reach sites of disease. There is need to modify drugs in a way that increases their half-life in the body. Some drugs, such as insulin, are easily degraded by digestive enzymes if orally ingested without a shield, hence the importance of a protective shell that can be easily formulated in the form of nanoparticles [1].
A large percentage of nano-particles used in drug delivery are natural molecules, such as proteins and polysaccharides. Most of them are already in the body, so biocompatibility is very high. There are other FDA approved polymers such as Poly(lactic) acid, poly(galactic) acid and their co-polymer, Poly(lactic-co-glycolic acid) which are also under increasing study for drug delivery due to their high solubility, biodegradability and response to stimuli such as pH in vivo [2].
History
Polymeric nanoparticles became more popular in the late 1960s, when Frank Davis invented the concept of PEGylation, which involved conjugating poly(ethylene) glycol and drug molecules to improve circulation and stability in vivo. In 1975, professor Helmut Ringsdorf sketched a drug conjugate made with poly(hydroxypropyl methacrylamide) and doxorubicin. It was synthesized in collaboration with Dr Ruth Duncan and James Cassidy in Prague. In 1984, a Japanese scientist named Hiroshi Maeda discovered the Enhanced Permeation and Retention Effect (EPR). He discovered that the vasculature in tumor cells is not completely developed, therefore they are leaky and nanoparticles can easily accumulate in tumor cells. The late 1980s and early 1990s saw the advent of micelles and liposomes as drug delivery nanoparticles. In 1995, Martin Woodle and Frank Martin’s liposomal formulation, involving PEG and doxorubicin, a cancer drug in the form of a product called Doxil was approved by the FDA. [3] In the 2000s, the protein based nanoformulations were adopted. Abraxane, a drug comprised of albumin ecapsulated placlitaxel for cancer treatment was approved by the FDA in 2005 for treatment of breast cancer. In 2013, it was approved for treatment of pancreatic cancer [4].
Polymeric Micelles


The study of polymeric micelles in drug delivery has been of increasing interest due to their advantages such as increased serum stability in the blood [1] and efficiency in transporting hydrophobic drugs. Because of their highly hydrophobic interior, micelles can fully encapsulate hydrophobic molecules and easily navigate the body’s aqueous transport environment with their hydrophilic exterior.
Micelles have been shown to successfully transport protein based drugs, such as bortezomib, a dipeptidyl boronic acid derivative used in treating multiple myeloma. These drugs act as proteasome inhibitor drugs in cancerous cells [2]. Proteasome inhibitors disrupt cell growth and differentiation therefore they must be delivered specifically to tumor cells, to prevent damage to healthy cells. Most peptide-based drugs easily lose their activity due to in vivo oxidation and have very poor bioavailability. Encapsulation in micelles provides a protective shell, aiding targeted delivery in response to triggers such as pH and temperature [1]
The main advantage in the application of polymeric micelles in cancer treatment is the enhanced permeation and retention effect (EPR), where Nanomolecules are easily entrapped within tumor cells which lack a fully developed lymphatic drainage system and are therefore leaky. [3] Because normal cells have an excellent drainage system, drug carrying micelles will not accumulate in them, further enhancing targeted delivery. The delivery of drugs through taking advantage of EPR in tumor cells is a form of passive targeting. [5].
Polymeric micelles are usually comprised of micelles and other linear co-polymers, such as linear poly(ethylene) glycol(PEG) and polyaminoacids such as poly(hydrazinyl)aspartamide. The linear polymers are conjugated with drug molecules through covalent bonding. The drug-polymer complex is then introduced into micelles through simple self assembly in aqueous media, which is usually water [2]. MG132 is a peptide aldehyde used in protein inhibition. Research has shown successful loading of MG132, with a loading efficiency of up to 75%. Polymeric micelles have been shown to successfully transport cisplatin and paclitaxel in animal studies. Clinical trials are currently underway to fully adopt them in targeted delivery of cisplatin in cancer therapy [2].
Polysaccharides as Drug Eluting Polymers
Chitosan
Chitosan is a naturally occurring cationic aminopolysaccharide which is obtained from the deacetylation of chitin, a carbohydrate based polymer. It is composed of N-acetylglucosamine and glucosamine, linked through a 1-4 glycosidic linker [1]. Chitosan’ s main molecular chain is hydrophilic, but also possesses hydrophobic behavior due to the presence of N-acetyl groups. As a result; chitosan tends to form aggregates difficult to dissolve in neutral conditions. Because of the presence of amino groups, chitosan is a natural polyelectrolyte therefore it can easily dissolve in acidic conditions [6]. As a result, chitosan can be easily applied in pH sensitive drug delivery, especially in the treatment of cancer, since tumor sites are acidic. Chitosan adheres to mucosal surfaces and can penetrate tight junctions, which makes it an excellent drug-eluting polymer. [1]Chitosan has been successfully used to orally deliver insulin, which is usually digested by enzymes in the gut before it can make it to the bloodstream [6]. Chitosan can easily form micelles, via self assembly due to its hydrophobic and hydrophilic compositions. This characteristic enhances its use as a delivery polymer for hydrophobic drugs.
Hyaluronic Acid
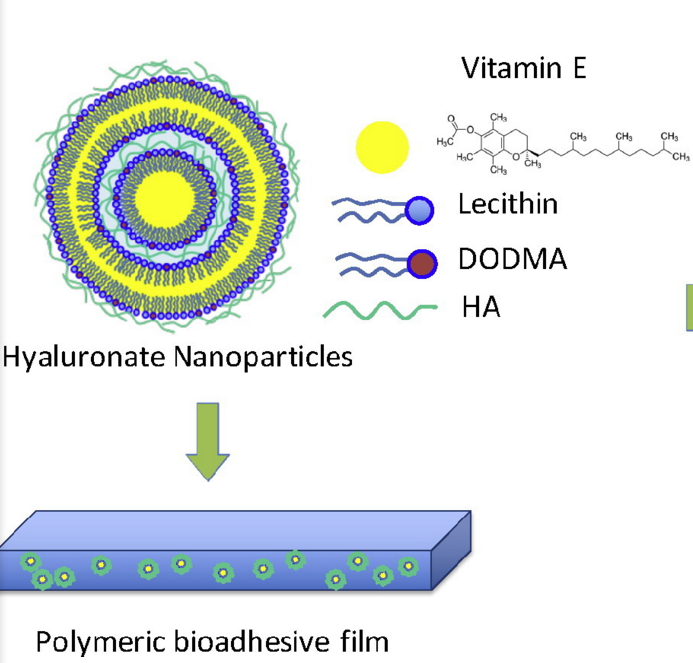
Hyaluronic acid (hyaluronate) is a naturally occurring polysaccharide, present in the extracellular matrix in the human body. It is highly soluble and can be easily modified for drug delivery, especially through conjugation with drug molecules and proteins. Hyaluronate has a very high water retention capacity; as a result it is popularly used in skin regeneration therapy, and is available commercially in anti aging creams from companies such as instaNatural. Hyaluronate has been successfully used in the controlled, slow release of Vitamin E for wound healing. Hyaluronic acid is applied in the synthesis of the nanoparticle itself, together the cationic lipid dioctadecyldimethylammonium bromide (DODMA) and lecithin. Hyaluronic acid is also incorporated in the synthesis of the polymeric film that holds the suspended nanoparticles, together with aloe Vera extract.
Hyaluronate is negatively charged; therefore it binds to the polar head of lipids through attractive electrostatic interactions. The hydrophobic portions of lipids interact with the lipophilic vitamin E. This is how the core shell of most particles involving hyaluronate and lipids are formed [7].
Proteins in Drug Delivery
Apart from their bioactivity, proteins have been increasingly under study for their application in drug delivery, especially in cancer treatment. Because of the protein abundance in the body, they are highly biocompatible. Albumin based nanoparticle carriers are currently being studied, after the FDA first approved Abraxane, albumin bound placitaxel for treatment of breast cancer in 2005. Abraxane was first developed by VivoRx in 1993 and is currently owned and produced by Bristol-Myers Squibb [4]. Placitaxel is a hydrophobic drug used in the treatment of ovarian, breast lung and other cancers. Because of its insolubility, It has a low bioavailability, of only 6.5% when orally ingested [10] hence the need for a drug carrier system.
Synthesis
The albumin-bound nanoparticle technology, developed first by Abraxis Bioscience is used in the safe, solvent free synthesis of nanoparticles [9]. Albumin is used to transport cancer drugs such as paclitaxel and doxorubicin, as well as tumor necrosis factor apoptosis inducing ligands [10]. In paclitaxel delivery, albumin is not covalently bound to paclitaxel, but rather through hydrophobic interactions. The paclitaxel nanoparticles are in a non-crystalline state, allowing for a speedy release in vivo. The albumin-paclitaxel molecule is then added to an 0.9% sodium chloride solution, before being injected into the body. In vivo delivery, especially in the treatment of cancers, is enhanced by SPARC (Secreted Protein, Acidic and Rich in Cysteine), a secreted glycoprotein that is over expressed by most tumor cells and has the ability to bind to albumin [9]. Albumin has been proved to show a steady release when studied in mice [10].
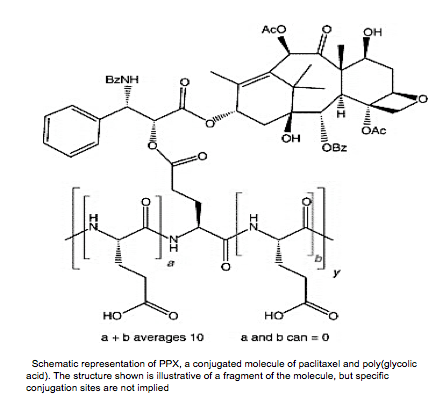
Apart from albumin, other peptide-based polymers are also used in drug delivery. One commonly used FDA approved polymer is poly-L-glutamic acid (PGA), a highly charged polyanionic peptide. PGA binds to most drug molecules through covalent bonding. PGA is advantageous because it is degraded to become poly(glutamic) acid, which easily enters cell metabolism. It is also highly soluble, even when conjugated up to 40% [11]. In the transport of paclitaxel, PGA is bonded to paclitaxel through an ester linkage on the carboxylic acid side chain of PGA [11].
The release mechanism for most of the peptide bound drugs in cancer treatment is through endocytosis. Because of the EPR effect, these large molecules will selectively target cancer cells, which are more permeable and have a high paucity which supports retention[3]. Upon arrival on the tumor site, the drug-polymer conjugate is absorbed through endocytosis, followed by intracellular release of the drug through the breakdown of the peptide backbone by lysozyme. [11].
Nanogels
Polylactic acid (PLA), poly-glycolic acid (PGA) and their copolymer Poly (lactic-co-glycolic acid) (PLGA) and Poly(ethylene)glycol are the most commonly used nanogels in drug delivery. They are among the few FDA approved formulations due to excellent biocompatibility. PLGA is easily broken down to give lactic acid and galactic acid, that are metabolized by the body in the Krebs cycle hence it is attributed with low toxicity and great biocompatibility.
The emulsification-solvent evaporation technique is often used in preparing PLGA nanoparticles. With this technique, the polymer and the compound are dissolved in an organic solvent such as dichloromethane. Encapsulation of hydrophobic drugs then follows. To prepare the emulsion oil in water, water and a surfactant, for example polysorbate-80 are added to the polymer solution. Sonication or homogenization is the two most commonly used methods for inducing nanosized particle formation. The solvent is then evaporated or extracted and the nanoparticles collected after centrifugation. Other techniques such as spray drying can also be incorporated in the place of evaporation. [12].
The most promising application of PLGA based nanoparticles is in the delivery of drugs across the blood brain barrier, which is the most difficult to penetrate. Research has shown that PLGA, because of its chemical structure can be conjugated to glycoproteins such as Lactoferrin, which have receptors on the blood brain barrier. Urocortin, a crucial molecule in combating Parkinson’s disease was successfully delivered to the brain through the combined use of both PLGA and Lactoferrin [13].
The body generally tends to treat hydrophobic molecules as foreign, hence there is need to coat hydrophobic drugs with hydrophilic molecules, hence the adoption of poly(ethylene) glycol, another FDA approved biopolymer for drug delivery. PEG improves the half-life of drugs significantly, and solves the challenge of high burst release associated with the use of PLGA alone. A PEG molecule can be easily incorporated into drug molecules due to the reactivity of both the ether and hydroxyl groups on the molecule. Furthermore, PEG forms conjugates with acid sensitive linkers before a PEGylation with any drug molecule. As a result, it can be used in the cancer targeting, for example in the transport of doxorubicin. Because PEG is known mostly for improving circulation time and shielding molecules from degradation, it is usually used in combination with other drug carrier systems, such as liposomes.
Costs and Funding
It is difficult to assign a specific market price to the polymeric nanoparticle industry. This is because of the diversity of polymers and diseases that they cure. Nanoparticles are used in a wide variety of health applications such as protein delivery, gene therapy, chemotherapy, lung, liver and brain diseases. Cancer research spending alone reached 100Billion worldwide in 2014, according to the IMS Institute for Healthcare Informatics, much of it being generally provided by the National Health Institute for research conducted in the United States.
Challenges
Regardless of the advantages posed by most drug eluting polymers, there are still some problems that still need to be overcome. PLGA, regardless of its minimal toxicity, has very low drug loading capacity [14]. PLGA also has the problem of high burst release, which makes it difficult in a lot of cases to reach target cells. Most synthesis methods used, such as sonication and ultracentrifugation are still challenging to scale up on an industrial level. Polymers such as PEG, though biocompatible are non-biodegradable therefore pose toxicity threats in high concentrations and molecular weights. In the formulation of most drug-nanoparticle complexes, some of the reagents such as dichloromethane that are used are toxic and may induce inflammation in the body
References
1.Karimi, M. Smart Internal Stimulus-Responsive Nanocarriers For Drug and Gene Delivery. 2015, 10–113.
2. Quader, S.; Cabral, H.; Mochida, Y.; Ishii, T.; Liu, X.; Toh, K.; Kinoh, H.; Miura, Y.; Nishiyama, N.; Kataoka, K. Selective intracellular delivery of proteasome inhibitors through pH-sensitive polymeric micelles directed to efficient antitumor therapy. Journal of Controlled Release 2014, 188, 67–77.
3.Hoffman, A. S. The origins and evolution of “controlled” drug delivery systems.Journal of Controlled Release 2008, 132 (3), 153–163.
4. Patent https://www.google.com/patents/US5439686
5. Biswas, S.; Kumari, P.; Lakhani, P. M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. European Journal of Pharmaceutical Sciences 2016, 83, 184–202. 6.Yang, Y.; Wang, S.; Wang, Y.; Wang, X.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnology Advances2014, 32 (7), 1301–1316. 7.Pereira, G. G.; Detoni, C. B.; Balducci, A. G.; Rondelli, V.; Colombo, P.; Guterres, S. S.; Sonvico, F. Hyaluronate nanoparticles included in polymer films for the prolonged release of vitamin E for the management of skin wounds. European Journal of Pharmaceutical Sciences 2016, 83, 203–211.
8. Peltier, Sandra; Oger, Jean-Michel; Lagarce, Frédéric; Couet, William; Benoît, Jean-Pierre (2006). "Enhanced Oral Paclitaxel Bioavailability After Administration of Paclitaxel-Loaded Lipid Nanocapsules". Pharmaceutical Research 23 (6): 1243–50. doi:10.1007/s11095-006-0022-2.PMID 16715372
9http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/WarningLettersandNoticeofViolationLetterstoPharmaceuticalCompanies/UCM289191.pdf
10.Thao, L. Q.; Byeon, H. J.; Lee, C.; Lee, S.; Lee, E. S.; Choi, Y. W.; Choi, H.-G.; Park, E.-S.; Lee, K. C.; Youn, Y. S. Doxorubicin-Bound Albumin Nanoparticles Containing a TRAIL Protein for Targeted Treatment of Colon Cancer. Pharm Res Pharmaceutical Research 2015, 33 (3), 615–626.
11.Singer, J. W. Paclitaxel poliglumex (XYOTAX™, CT-2103): A macromolecular taxane. Journal of Controlled Release 2005, 109 (1-3), 120–126.
12.Di-Wen, S.; Pan, G.-Z.; Hao, L.; Zhang, J.; Xue, Q.-Z.; Wang, P.; Yuan, Q.-Z. Improved antitumor activity of epirubicin-loaded CXCR4-targeted polymeric nanoparticles in liver cancers. International Journal of Pharmaceutics 2016, 500(1-2), 54–61.
13. K. Hu, J. Li, Y. Shen, W. Lu, X. Gao, Q. Zhang, X. Jiang, Lactoferrin-conjugated PEGPLA nanoparticles with improved brain delivery: in vitro and in vivo evaluations, J. Control. Release 134 (2009) 55–61.
14 Danhier, F.; Ansorena, E.; Silva, J. M.; Coco, R.; Breton, A. L.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release 2012, 161 (2), 505–522.
15. www.bustle.com
16.www.blueberrytherapeutics.com/nanomedicine/nanopolymer-drug-delivery-systems
