Fibrous Gel, by Singyuk Hou
Introduction
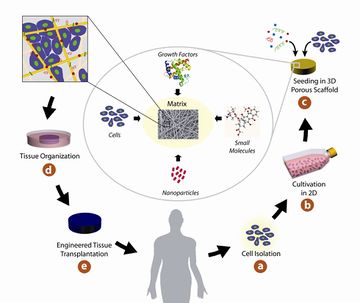
In tissue engineering, gel or scaffold serves as artificial material to support and guide three-dimensional growth of cells into functional tissues or organs. Fibrous gel is the type of scaffold that contains fibrous network (1). To enable cell growth, there are common requirements for designing the scaffolds. The most important criteria is biocompatibility, the material should not be toxic to the cells or induce severe immune responses to the recipients of the scaffold. Second, the scaffold should interact with cell positively to enhance cell adhesion, migration, differentiation and growth. Third, the scaffold should have sufficient mechanical integrity, so that predesigned tissue structure can be maintain after implantation. Forth, biodegradability is needed to be considered as proper degradation rate is needed to match the formation of new tissue. Fifth, scaffolds have to be highly porous to facilitate cell ingrowth and homogeneous distribution of cells and formation of new vasculatures (2).
Motivation
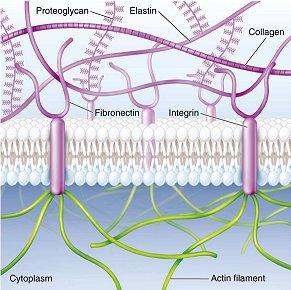
The development of fibrous gel or scaffold is the result of mimicking extracellular matrix, since it is the natural environment for cells to proliferate, differentiate and function (3). Extracellular matrix mainly consists of water, proteoglycans and fibrous proteins. Proteoglycans fill the extracellular interstitial space in the form of highly hydrated gel. Fibrous proteins including collagens and elastins provide structural and biochemical support to the surrounding cells. For example, collagens provide tensile strength of tissue and regulate cell adhesion; elastins provide recoil to tissues so that they can resume their shape after stretching. In addition, fibronectins direct the development of extracellular matrix and mediate cell attachment on protein fibrils generated by collagens (4).
Since mammalian cells can sense their local environment through cell receptors, for example, RGD peptide sequence on fibronectin can be recognized by integrins, therefore, to design scaffolds similar to the fibrous proteins in extracellular matrix is a rational approach (3).
History

Scaffold has been used for over 25 years and has evolved during the process of optimization. The first report on using artificial materials to culture cells was published in 1988 by Vacanti (5). One year later, Wakitani reported the use of chondrocytes embedded in collagen gel to accelerate healing of injured rabbit kneecaps (6).
Materials
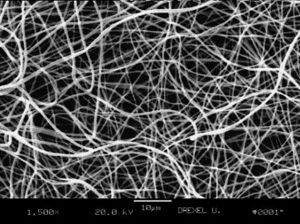
The materials used to fabricate fibrous gel are usually polymeric, the reason is due to their unique properties such as high surface-to-volume ratio, high porosity, biodegradability, and mechanical property (7). The materials can either be natural or synthetic. While nature-derived polymers provide better bioactivity to cells, there are more challenges in quality control and scale-up production. Synthetic materials on the other hand can compensate those drawbacks (8). The list below encampus the most commonly used materials nowadays.
Natural
Collagen
Collagen is the most abundant protein found in extracellular matrix. Commonly, three collagen helices organized themselves into fibril bundles. Those bundles provide the tissue with tensile strength and regulate binding of other components that cause cell adhesion and growth. Different types of collagens can be found in different tissues, among those the most common one is type one collagen, it can be found in dermis, bone, organ capsules, tendon and so forth. Since collagen can be obtained from a large range of sources, it is widely used to form tissue engineering scaffolds. However, the potential risk of using collagen is contamination from pathogens. Also, chemical cross-linking is needed to make collagen based scaffolds have enough mechanical strength, which may need to conduct with detrimental solvent. Fast degradation also limit the use of collagen scaffold (8).
Elastin
Elastin consists of alternating hydrophobic regions and cross-linking domains, so it is not water soluble. The structure provides tissue elasticity and recoil. Elastin presents in a artery, lung, skin and intestines. The drawback in using elastin in tissue engineering includes difficulties in purification (8).
Silk
Silk is fibrous protein produced by insects and spiders. It consists of two proteins, sericin and fibroin. Silk is considered to be nondegradable material and it has been documented as suture due to its excellent tensile mechanical properties (8).
Synthetic

PLA (Poly (lactic acid))
PLA is a biodegradation linear aliphatic polyester. Due to the higher tensile strength and slower degradation rate comparing to poly(glycolic acid), PLA is more suitable for load-bearing application, such as orthopedic fixation. Degradation product of PLA is lactic acid which can be readily metabolized by body (9).
PGA (Poly (Glycolic acid))
PGA is another biodegradation linear aliphatic polyester. It is first developed and commercialized in 1970s as biocompatible suture. PGA has glass transition temperature comparable to physiological temperature, therefore loss of mechanical strength may occur after implantation in human body. Degradation rate of the material is faster than PLA, because glycolic acid is a more hydrophilic building block. Degradation product of PGA is glycolic acid (9). However, since glycolic acid is not readily metabolized by human body, there are more reports on adverse reactions after implantation,such as swelling and pain(10).
PLGA (Poly (Lactide-Co-Glycolide))
PLGA is the copolymer formed by PGA and PLA. It exhibits lower crystallinity and melting temperature comparing to PLA and PGA. The rate of degradation can be adjusted by the ratio of PLA/PGA. Product of degradation is mixture of lactic acid and glycolic acid (9).
Fabrication
The conventional extrusion methods have difficulty in generating fiber with diameter less than 10 μm, which is 1 to 2 magnitude larger than that of protein fiber bundles in extracellular matrix (50-500 nm). Therefore, several methods were developed to generate nanoscale fibers(11).
Electrospinning

Electrospinning is a well-established process to generate fine fibers by applying voltage between polymer solution or melt and grounded target. Polymer melt or solution is drawn from a capillary or needle to form a suspended droplet at the tip of the capillary or needle . The polymer jet is initiated toward the collector target when the electrostatic charge overcomes the surface tension of the droplet. This result in continuous non-woven fiber mat (11). The diameter of the fiber can be controlled by tuning surface tension of polymer solution (melt), flow rate from capillary or needle, and electric current (12). There are several attractive aspects using this method, for example, by changing the orientation of the polymer fiber, the mechanical properties can be adjusted. The simplicity of the method facilitates mass productio. The drawback of the method is usage of toxic solvent (13).
Self-assembly
Self-assembly involves the independent organization of individual components into functional structure with predesigned non-covalent bonds. The most common configuration to produce nanofiber is peptide-amphiphiles(PA) (11). PAs consist of five essential building block: long alkyl chain, providing hydrophobic drive in aqueous solution; four consecutive cysteine residues to create disulfide bonds for polymerization (14). In aqueous phase, long alkyl chain cluster to form cylindrical micelle followed by formation of intermolecular disulfide bonds between cysteine residues. This process allow formation of 5-8 nm nanofiber (11). This methold however, only applicable to a narrow scope of materials.
Phase separation
Phase separation happens in polymer solution, which can be induced thermally or by adding nonsolvent to solution to create a gel. Water is then used to extract the solvent from the gel and the gel is cooled to a temperature below the glass transition temperature of the polymer and freeze dried under vacuum to produce a fibrous scaffold. By addition of porogens in phase separation process, the pore size and interconnectivity of the material can be controlled easily. And the interfiber distance can be controlled by tuning the concentration of polymer solution. This method is also reported to have good batch-to-batch consistency (11). However, same as electrospinning method, the solvent used can also be detrimental to cells (13).
Future of Fibrous Gel
Presently, emphasis is placed on the design of polymeric scaffold, which has attracted attention from multiple disciplines. However, the progress from lab to clinical application is limited. The factors that could help translation to clinical application include to work closely with end users and surgeons to understand material design requirements so that surgical acceptance of new materials can be increased,to develop scalable methods and to integrate computational design to fabricate materials with three-dimensional structures, to understand more comprehensively about how cells interact with scaffold materials and to develop screening methods to efficiently find out factors that can improve the design of scaffold materials (15),(16) and (17).
References
[1]Chen, G., Ushida, T. & Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2, 67–77 (2002).
[2]Ma, P. X. Scaffolds for tissue fabrication. Mater. Today 7, 30–40 (2004).
[3]Dvir, T., Timko, B. P., Kohane, D. S. & Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 6, 13–22 (2011).
[4]Frantz, C., Stewart, K. M. & Weaver, V. M. The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 (2010).
[5]Vacanti, J. P. et al. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J. Pediatr. Surg. 23, 3–9 (1988).
[6]Wakitani, S, T Kimura, A Hirooka, T Ochi, M Yoneda, N Yasui, H Owaki, and K Ono. “Repair of Rabbit Articular Surfaces with Allograft Chondrocytes Embedded in Collagen Gel.” The Journal of Bone and Joint Surgery. British Volume 71 (1989): 74–80.
[7]Place, E. S., George, J. H., Williams, C. K. & Stevens, M. M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 38, 1139–51 (2009).
[8] Wolfe, Patricia. S, Sell, Scott. A, and Bowlin, Gary. L. (2010). “Chapter 3: Natural and synthetic scaffolds” In Norbert Pallua and Christoph. V. Suscheck (Ed.), Tissue Engineering: From Lab to Clinic (pp.41-68). Berlin, Heidelberg: Springer Science & Business Media.
[9]Dhandayuthapani, B., Yoshida, Y., Maekawa, T. & Kumar, D. S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 1–19 (2011).
[10]Ambrose, C. & Clanton, T. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann. Biomed. Eng. 32, 171–177 (2004).
[11]Smith, L. A. & Ma, P. X. Nano-fibrous scaffolds for tissue engineering. Colloids Surfaces B Biointerfaces 39, 125–131 (2004).
[12]Fridrikh, S., Yu, J., Brenner, M. & Rutledge, G. Controlling the Fiber Diameter during Electrospinning. Phys. Rev. Lett. 90, 144502 (2003).
[13]Hartgerink, J. D., Beniash, E. & Stupp, S. I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294, 1684–1688 (2001).
[14]Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 3, 589–601 (2006).
[15]Hollister, S. J. & Murphy, W. L. Scaffold translation: barriers between concept and clinic. Tissue Eng. Part B. Rev. 17, 459–74 (2011).
[16]Hollister, S. J. Scaffold design and manufacturing: from concept to clinic. Adv. Mater. 21, 3330–42 (2009).
