Grierson Lab:Further information: Difference between revisions
No edit summary |
mNo edit summary |
||
| Line 23: | Line 23: | ||
=== '''Objective 2''' === | === '''Objective 2''' === | ||
Understanding when and how root hair formation is triggered. Auxin transport is a key regulator of RH development. Each new RH cell elongates (along the axis of the root) as it moves away from the root tip, before producing a single root hair. The position on the root where RHs form is influenced, inter alia, by auxin-responsive transcription factors. New results from the Grierson lab (Fig. | Understanding when and how root hair formation is triggered. Auxin transport is a key regulator of RH development. Each new RH cell elongates (along the axis of the root) as it moves away from the root tip, before producing a single root hair. The position on the root where RHs form is influenced, inter alia, by auxin-responsive transcription factors. New results from the Grierson lab (Fig. 3) show RH cells express very different levels of the auxin influx carrier AUX1 from non-hair cells. Interpretation based on Kramer’s [http://www.simons-rock.edu/~ekramer/] model of auxin flow through the root suggests this should result in root hair cells containing very different levels of auxin from non-hair cells. We are testing this prediction by measuring the auxin content of hair and non-hair cells in collaboration with Ljung [http://static.upsc.se/kljung.htm]. [[Image:Root_hair_cross_section.png|frame|Figure 2. Cross-section of an Arabidopsis root tip showing the two types of cell in the epidermis. RH cells (yellow) overly the junction between two cortical cells in the layer below, whereas non-RH cells (blue) overlie a single cortical cell. Specific transcription factors establish this pattern by moving between the cell types and regulating each other’s activity.]]We are also ascertaining the contributions that auxin and ethylene make to hair development using a combination of hormone treatments, fluorescent imaging, genetic manipulation, and measurements of auxin content and response. In parallel we will build a mathematical model of hair morphogenesis using modified reaction-diffusion equations, and test whether the robustness of the morphogenesis is explained by a Turing-like process. Preliminary results from Dolan’s lab [http://www.jic.bbsrc.ac.uk/science/cdb/dolanWebpage.htm] indicate that the ability of auxin and ethylene to trigger root hair formation depends on two new transcription factors, whose relation to the transcription factors alluded to in Figure 1 is being examined using microarray data, transgenic reporters, triple mutants and hormone treatments. Bioinformatics tools could identify similarities between the promoter sequences of putative targets and suppressors, look for likely transcription factor binding sites, and identify possible mechanistic links with auxin and ethylene. The results will also feed into Objective 3. | ||
Revision as of 06:33, 10 January 2007
Major new collaborations are being established and are summarised below (website still in constuction). In the mean time you can learn about our current work on the Lab Members and Research pages.
Summary
We are pioneering an integrative, predictive biology approach to describing the mechanisms of differentiation, morphogenesis and directed elongation of an individual cell. Root hair (RH) cells of Arabidopsis are an ideal model for this because their development is exceptionally well understood and they are relatively easy to study experimentally. This project brings together a range of life and theoretical scientists to piece together our current knowledge and use novel experiments, mathematical models, and biocomputation to begin to understand the system as a whole. This work will contribute to the global research effort on a “virtual” plant by linking to the virtual root model being developed at the Centre for Plant Integrative Biology (CPIB) at Nottingham (Nottingham CPIB homepage). In addition the project will develop insights and approaches relevant to a wide range of biological systems, and boost our understanding of complex systems in general.
Root hair development presents a unique set of challenges that are beyond the scope of the CPIB, and are outlined here. The research builds on skills already at Bristol: a world-leading root hair biology group (Grierson Lab frontpage), expert mathematical modelling in dynamical systems (Prof. Champneys, University of BristolDr Payne, University of Bristol), mechanics (Dr Chenchiah, University of Bristol), bioinformatics (Dr Gough, Institut Pasteur Paris), and computational (Prof. Flach, University of Bristol), and statistical (Prof. Green FRS, University of Bristol) modelling plus leading edge techniques in light (cell imaging facilities, University of Bristol) and atomic force (Prof. Miles, University of Bristol) microscopy and image analysis (Dr Mirmehdi, University of Bristol). Other UK expertise in modelling and experimentation at Sheffield (Dr Monk, University of Sheffield), Norwich (Prof Dolan, John Innes Centre) and Nottingham (Nottingham CPIB homepage).
Rationale
Root hairs are agronomically important. They make up the majority of the root surface area of many crops, where they play an essential role in taking up nutrients and water from the soil, in interacting with pathogens and symbionts, and in anchorage. Research into optimisation of root hair properties is vital, since current agricultural usage levels of fertiliser and fresh water are not sustainable. Historically root hair research has been multidisciplinary and has involved developmental, genetic and cellular approaches to investigate the network controlling formation of the cell. More is known about the mechanism underpinning root hair cell development than any other plant cell type. Hair cells are an exemplary experimental system: they develop in a predictable spatial pattern (Fig.1), allowing cells to be imaged throughout their development, and they develop cylindrical “hairs” that grow away from the surface of the plant into surrounding medium, and which are transparent thus facilitating quality imaging. Genetic knowledge of root hairs is excellent and there are many viable mutants and transgenic lines available, along with other outstanding international resources. These mutants are often characterised by specific aberrant morphologies, and the ability to explain these mutant forms will be a key bench-mark of our work. Root hairs are also an outstanding system for generic modelling of plant cell development differentiation and growth, posing a series of profound biological questions that are relevant to many other biological systems.

Objectives
We will focus on six objectives.
Objective 1
Understanding which epidermal cells become root hair cells. Epidermal cells differentiate into RH and non-RH cells in a predictable pattern (Fig. 2), which has yet to be understood in a context that permits an understanding of root auxin flow and RH outgrowth. Monk & Dolan have developed an ordinary differential equation model for the gene expression and cell-cell movement of transcription factors that control epidermal cell differentiation. We will add a longitudinal dimension to these models to address how the assembly of the transcription factor gene network might interact with the transport of long-range regulatory molecules, like auxin. This novel multi-level modelling approach is needed because key regulators such as ethylene and auxin are known to modify, and sometimes even to prevent manifestation of the RH and non-RH phenotypes.
Objective 2
Understanding when and how root hair formation is triggered. Auxin transport is a key regulator of RH development. Each new RH cell elongates (along the axis of the root) as it moves away from the root tip, before producing a single root hair. The position on the root where RHs form is influenced, inter alia, by auxin-responsive transcription factors. New results from the Grierson lab (Fig. 3) show RH cells express very different levels of the auxin influx carrier AUX1 from non-hair cells. Interpretation based on Kramer’s [1] model of auxin flow through the root suggests this should result in root hair cells containing very different levels of auxin from non-hair cells. We are testing this prediction by measuring the auxin content of hair and non-hair cells in collaboration with Ljung [2].

We are also ascertaining the contributions that auxin and ethylene make to hair development using a combination of hormone treatments, fluorescent imaging, genetic manipulation, and measurements of auxin content and response. In parallel we will build a mathematical model of hair morphogenesis using modified reaction-diffusion equations, and test whether the robustness of the morphogenesis is explained by a Turing-like process. Preliminary results from Dolan’s lab [3] indicate that the ability of auxin and ethylene to trigger root hair formation depends on two new transcription factors, whose relation to the transcription factors alluded to in Figure 1 is being examined using microarray data, transgenic reporters, triple mutants and hormone treatments. Bioinformatics tools could identify similarities between the promoter sequences of putative targets and suppressors, look for likely transcription factor binding sites, and identify possible mechanistic links with auxin and ethylene. The results will also feed into Objective 3.
Objective 3
Understanding where on each cell hair outgrowth is initiated. At the beginning of root hair growth, an ellipse of bulging and thinning wall about 20 microns long and 10 microns across is produced in approximately 2 hours at the end of the cell nearest the root tip (independent of alignment with gravity; Fig 3)
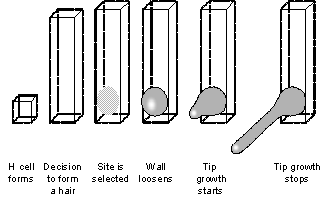
. Previous work has shown that major roles are played by RHD6 [4], RHD1 UDP-glucose 4-epimerase [5][6][7], TIP1 S-acyl transferase [8], and ROP small GTPases [9][10] [11] and their negative regulator ROPGDI1 [12]. In order to investigate the mechanisms by which these factors might operate, we will build a mathematical model that describes the flow of auxin through and around the cell, the changes in cell wall properties and how these might affect the shape of the bulge, and the interior structure and networks within the cell (using approaches outlined in Objective 4 Strand a) that influence cell wall properties. In tandem we will closely investigate changes in these properties using Miles's expertise in AFM microscopy of biological tissue. Miles has already observed reproducible changes in the “springiness” of the cell wall at the growth site. This work will be complemented by studies with a turgor pressure probe and imaging system being developed by Tansley at CPIB [13]. This probe can measure turgor pressure, and reveal changes in local cell wall properties by observing and recording how the wall deforms when the turgor pressure within a given cell is increased. For example, local wall loosening or softening at the bulge site might produce a patch of wall that will bulge out more as turgor is increased. This work will contribute useful data to our integrative approach of experimentation, imaging, and development of biomechanical models of the cell wall.
Objective 4
Understanding how root hair elongation and shape is controlled. A model of hair elongation must take into account various experimentally measured features, e.g., relative time scales associated with initiation, growth and chemical (including auxin) transport within the root hair; clarify the behaviour of at least some genetic mutants, e.g., those with branched, wavy or corkscrew hairs; and explain different morphological forms associated with changes in the internal or external environments. Broadly speaking, modelling will fall into two strands: Strand (a). Molecular machines involved in tip growth. We will investigate the transcriptional control that accompanies root hair growth using data generated under Objectives 1 and 2 and additional data on further transcription factors that maintain root hair elongation (Dolan, unpublished). In addition we will use approaches developed from those of Gunsalus et al. model the network of genes and gene products that contribute to the molecular machines involved in growth using microarray data, bioinformatics, and high throughput phenotyping methods to be developed collaboratively by Mirmehdi, Leica, and Pridmore at CPIB. By combining these analyses we will gain insights into the way that the growth process develops, and the order in which the root hair growth machinery accumulates in the cell. Up to a third of the tip growth network consists of genes of hitherto unknown function (Breen and Grierson, unpublished). A network analysis will enable us to predict functions for many of these genes by identifying their association in the network with genes of known function. Our results will help to launch work on the evolution, structure, function, and organisation of tip growth networks (including those in fungal hyphae, pollen tubes, and nerve cells). The models will also incorporate spatial information about the subcellular localisation of components, essential for understanding development and growth, but not commonly included in cell network models. Strand (b). Biomechanics of growth. Since root hairs grow in an approximately straight line we propose beginning with a 1D growth model which will be extended to 2- or 3D. These will be augmented with models for the cell wall and for the fast diffusion of the necessary cell-wall building blocks along actin filaments, through vesicles and vesicle supply centres. These three component models would then be integrated to obtain 2D or 3D models for the biomechanics of the growing root hair. The experimental data required for the development of these models will be obtained as in Objective 3, focusing on the properties of newly deposited wall at the tip, and of the hardening wall surrounding it. Aberrant forms will be investigated through these models: e.g., we will investigate the consequences of faults in feedback control systems and positioning of vesicle supply centres -- oscillatory perturbations might lead to observed wavy morphologies. The causes of these faults can then be addressed through the molecular machines models developed in Strand (a) and by testing predictions about gene function in living plants.
Objective 5
Understanding how cessation of hair growth is controlled. The extent of root hair growth is agronomically important, since it controls efficacy of nutrient uptake. Mechanisms of growth cessation are tightly coordinated so that the end of the mature hair is a tidy dome-shape. Several mutants and treatments disrupt this process and produce distorted tips on mature hairs, such as hairs with bulbous, pointed, or branched ends. Two sets of questions arise. First, do local changes in auxin transport or levels affect this process? We will address this by collecting image data on the locations of auxin transporters in the region of the root where growth ceases and including the results in our models of auxin flow through the epidermis, and the auxin environment surrounding a developing hair cell. Secondly, how is the activity of the tip growth machinery stopped, and how is this coordinated? Our phenotyping of mutants in the genes of the tip growth network will include assays for phenotypes affecting the end of growth. Ultimately these conditions will be fed into the models arising from Objective 4 to seek a coherent explanation.
Objective 6
Understanding root hair development. To answer this requires consideration of the root hair cell within both the root and the plant as a whole. Our modelling approaches will be designed to enable integration into the Virtual Root model at CPIB and hence the international Virtual Plant. We will appoint a Scientific Computing Officer for the entire duration of the project to feed the various models arising from Objectives 1-5 into a unified software environment. This will enable virtual experiments to be done in silico that will help us to prioritise future theory work and experiments.
Project management and outreach.
At the moment the objectives are being researched as time and resources allow without dedicated funding and they are being managed separately by the relevant academics. We are applying for funding to appoint dedicated post docs for this research. This funding would also enable us to appoint people to help us to manage the projectand maintain strong coordination, data sharing, and software matching with Sheffield, Norwich and CPIB Nottingham.
The following have provisionally agreed to sit on an International Steering Committee: Philip Benfey (Root development, Duke University), Lalith Mahadevan (Biomechanics, Harvard), Anne-Mie Emons (Root hair cytoskeleton, Wageningen), Gabor Forgacs (Biological viscoelasticity, Missouri), Martin Howard (Intracellular dynamics, Imperial), Philip Maini (Mathematical Biology, Oxford), John Schiefelbein (Root hair development, Michigan).