Griffin:Western Blot Optimization
Your time and labor at the bench is extremely valuable. Below is a guide that will enable you to interpret your western blotting experience in a purposeful way so that each experiment will make a difference. Watch out for futile cycling in your work and focus your energy in a meaningful way.
There is an increasing number of available commercial antibodies from several vendors. Depending on who you buy from, the level of quality control the vendor performs is highly variable and this leaves you with the responsibility to optimize your own protocol in an efficient manner. Because every antibody is different, each one requires a different blocking/incbuation buffer in order to optimize the signal:noise ratio.
There are common themes in the types of incubation buffers that tend to work well for blocking and incubation.
Commercial antibodies have a wide range of specificity and sensitivity for the protein of interest. Effecient blocking of the primary and secondary antibodies can take place in a variety of different blocking agents that contain soluble proteins and nonionic detergent. When developing a new antibody by western blot, it is important to test several different blocking/incubation buffers for the best possible signal:noise ratio in the assay. No single blocking agent is ideal for every primary antiobody, since each antibody-antigen pair has unique binding characteristics.
The importance of the blocking/antibody incubation buffer
PVDF or nitrocellulose membrane has the ability to bind protein of all sorts. Since both the antibodies and the transferred lysate/extract are proteins, steps must be taken to optimze interactions between the membrane and the antibody used for detection of the target protein.
Blocking of non-specific binding is achieved by placing the membrane in a dilute solution of protein containing a low percentage of detergent such as Tween-20. The protein in the dilute solution attaches to the membrane in all places where the target proteins have not attached from the gel transfer to the membrane.
When the primary antibody is added, there is no room on the membrane for it to attach other than on the binding sites of the specific target protein. This reduces "noise" in the final product of the Western blot, leading to clearer results.
Band appearance/aesthetics
Smile effect of the bands/Dumbbell shaped bands
1. Migration was too fast.
2. Migration was too hot (changing the pH and altering the migration). Slow down the migration or run the gel in the cold room or on ice.
Uneven band size in lanes probed for the same protein
Gel has set too quickly while casting and the acrylamide percentage is not even along the lanes. Review the recipe of the gel and the addition of TEMED to the gels, add a little 0.1% SDS in water to the top of the migrating gel while it sets to stop it from drying.
Molecular weight disparity
Always review protein molecular weight details in the primary literature through PubMed for the most informed perspective on your result. Below are possibilities to explain the infinite nuances of protein behavior in SDS-PAGE.
Post-translation modification
- phosphorylation: Tyrosine, Serine, Threonine phosphoryl additions are common.
- glycosylation: Carbohydrate additions due to ER/Golgi processing can increase the weight of the protein.
- cleavage: pro-proteins undergo cleavage to render the active form.
mRNA splice variation
- Alternative splicing of exons can create different sized proteins from the same gene. Depending on cell type, differentiation state, & tissue type, the transcript splicing can and will influence structure and function for certain genes.
Intrinsic structure
- Relative charge: The composition of amino acids (charged vs non-charged).
- Multimers: Modular proteins or proteins than can form multimeric complex can influence the observable weight. This is usually prevented in reducing conditions through the use of DTT, 2-Mercaptoethanol, or TCEP. However, strong interactions can result in the appearance of higher bands.
Black spots on film

- Primary and/or secondary antibody aggregation in solution will cause the film exposure to appear speckled; either primary or secondary antibody. Antibodies may be sticking to the blocking agent.
Solution:
- Microfuge the primary and secondary at 14,000xG for 15 minutes at 4C.
- 0.2 micron filter sterilize the antibodies into a fresh sterile tube and relabel the tube.
- 0.45 uM filter the blocking agent.
- Poor Transfer due to air bubbles during transfer to membrane, dirty film when adding to the developer, developer rolls are dirty
Solution:
- use a pasteur pipette as a roller. roll the membrane and gel in the submersed transfer buffer before transfer to remove bubbles. keep membrane and materials wet.
- keep film clean before adding to the developer
Concentration of antigen
The resolution of SDS-PAGE is limited to 50-100 bands. If the relative concentration of the antigen of interest is too low (less than 0.2% of total protein), it may be difficult to detect (for instance, synaptobrevin/VAMP comigrates with histones in cell homogenates which interfere with its detection). Signal enhancement may then lead to the appearance of artificial bands. Enrichment of the antigen by fractionation or by immunoprecipitation should be considered.
Excess signal; multiple bands/too much banding
Theoretical prediction is a primary antibody toward a single gene product should produce a single band. While this is common and in some cases to be expected, there are legitimate exceptions to the rule and other factors may be responsible. For example:
Factors that can produce multiple bands of variable molecular weight
- transcript variation
- multiple start/stop sights
- signal-dependent protein processing/shuttling (ie secretion)
- cell type/differentiation state specific variation
- endoplasmic reticulum and golgi-dependent alternative cofactor additions
Smearing effect for the band of interest
- post-translational modifications including glycosylation, nitrosylation, phosphorylation, methylation, acetylation, ubiquitination,
- ubiquitin-dependent protein degradation
Excess signal; dark exposure
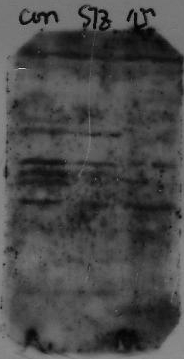
How do I decrease the background on my blot?
The leading cause of excess background is cross-reactivity between blocking agent and primary or secondary antibody: this will result in an overall membrane staining. The best blocking/incubation buffer for your immunoassay is the one that gives you the most clean bands with minimal background noise.
Concentration of antibody may be too high or incubation time too long. Also, several short washing steps are better than one long one.
Additional considerations;
The primary antibody you purchased may be too sensitive for specific detection of the target protein. Contact the vendor and explain your results. Value your time and notify the vendor of your observations. Common feedback may include;
1. Further dilute out your HRP or AP conjugated secondary antibody.
2. Instead of primary antibody incubation overnight, try 2 hours at room temp.
3. Instead of 2 hours at room temp, try 1 hour are room remp or 1 hour at 37C.
4. Perform more wash steps between incubation steps. Try 5 shake rinses followed by 4 x5min washes in 1X TTBS. Several short washing steps are better than one long one.
5. Load less protein onto the gel;
whole cell lysate or tissue extract: 20-50 micrograms subcellular fractions (ie nuclear or cytosol extracts): 10-30 micrograms purified proteins (ie recombinant or eukaryotic expression): 5-50 nanograms
- Blocking of non-specific binding might be absent or insufficient. Increase the blocking incubation period and consider changing blocking agent. *The primary antibody concentration may be too high. Titrate the antibody to the optimal concentration, incubate for longer but in more dilute antibody (a slow but targeted binding is best).
- Incubation temperature may be too high. Incubate blot at 4°C.
- The secondary antibody may be binding non-specifically or reacting with the blocking reagent. Run a secondary control without primary antibody.
- Cross-reaction between blocking agent and primary or secondary. Add a mild detergent such as Tween20 to the incubation and washing buffer.
- Antibody detects the casein present in the milk. Use BSA as a blocking reagent instead of milk.
- Washing of unbound antibodies may be insufficient. Increase the number of washes.
- Your choice of membrane may give high background. Nitrocellulose membrane is considered to give less background than PVDF.
- The membrane has dried out. Care should be taken to prevent the membrane from drying out during incubation.
Ghost bands (reverse/white banding)
Insufficient blocking
Ghost banding is the negative image of the proteins that have been transferred to the membrane.
Visualize in your mind; A 15 lane gel that has been run out and coommassie stained. Every lane will have a laddering appearance. Now imagine this same laddering band pattern being transferred to a PVDF or nitrocellulose membrane. The transfer of the proteins from the gel onto the surface of the membrane creates a protein fingerprint on the membrane. Blocking the membrane is an attempt to cover all other unbound sights.
Ghost banding occurs when there is residual background noise around where the protein fingerprint is present. This event suggests;
1. There is no detectable level of the target protein in the samples
2. The primary antibody is not recognizing the target protein
Excessive signal generated
Reduce the primary/secondary antibody dilution and lower the protein loading amount; Dilute HRP-conjugate at least 10-fold. High levels of specific antibody and an overabundance of protein can cause intense localized signals (typically a single band). Rapid and complete quenching of the substrate will produce no signal. Since there is no light production after the completion of the reaction, white bands are the result when exposed to film.
No signal

- Run an antibody control: parallel Actin or GAPDH positive control western blot that corresponds to the same host species as your experimental primary antibody will conclusively indicate if the issue relates to your protocol on some level OR an issue with the primary antibody/protein expression level. This also validates that your secondary antibody is functional.
- Run a positive control: Running a sample that is known to express your protein of interest will conclusively indicate if the issue relates to the primary antibody.
- Antigen is not recognized by primary antibody & this can occur especially with monoclonal antibodies that were raised against a native protein. In some cases, a non-reducing gel system may need to be used. Otherwise contact the vendor technial service.
1. Reagent omitted or improperly prepared. A simple fix yet this becomes more and more rare with experience. Review the protocol.
2. Protein did not transfer from gel to membrane. Try a Ponceau S stain of the membrane to see if there are bands on the membrane.
3. Specificity of HRP secondary antibody not appropriate for primary antibody.
4. Correct orientation of membrane not maintained throughout procedure.
5. Presence of azide in buffer, inhibiting peroxidase activity. Horseradish peroxidase labeled antibodies should not be used in conjunction with sodium azide. A change in the blocking agent or incubation solution will solve this problem.
6. Detergent is too harsh: SDS, Nonidet P-40, and Triton X-100 disrupt binding between proteins. 0.01-0.05% Tween-20 is the most commonly used and recommended detergent for washing and incubation solutions.
- The primary antibody and the secondary antibody are not compatible. Use secondary antibody that was raised against the species in which the primary was raised (e.g primary is raised in rabbit, use anti-rabbit secondary).
- Not enough primary or secondary antibody is bound to the protein of interest. Use more concentrated antibody. Incubate longer (e.g. overnight) at 4ºC.
- Cross-reaction between blocking agent and primary or secondary antibody. Use a mild detergent such as Tween20 or switch blocking reagent (i.e. commonly used blocking reagents are milk, BSA, serum or gelatin).
- The primary antibody does not recognize the protein in the species being tested. Check the datasheet or perform a ClustalW alignment to ensure your antibody should react with the target protein; Run the recommended positive control.
- Insufficient antigen. Load at least 20-30 ug protein per lane; Use protease inhibitors; Run the recommended positive control.
- The protein of interest is not abundantly present in the tissue. Use an enrichment step to maximize the signal (e.g. prepare nuclear lysates for a nuclear protein, etc.).
- Poor transfer of protein to membrane. Check the transfer with a reversible stain such as Ponceau S; check that the transfer was not performed the wrong way; if using PVDF membrane make sure you pre-soak the membrane in MeOH then in transfer buffer.
- Excessive washing of the membrane. Do not over wash the membrane.
- Too much blocking does not allow you to visualize your protein of interest. Instead of using 5% milk in the antibody buffers try removing the milk or using 0.5%; Switch blocking reagents or block for less time.
- Over-use of the primary antibody. Use fresh antibody as the effective concentration is lowered upon each re-use.
- Secondary antibody inhibited by sodium azide. Do not use sodium azide together with HRP-conjugated antibodies.
- Detection kit is old and substrate is inactive. Use fresh substrate.
Proteolytic breakdown of the antigen
If additional smear/ladder type banding is of lower apparent molecular mass than the full-length protein, then proteases may be active. The addition of fresh protease inhibitors such as PMSF, pepstatin or leupeptin can resolve this. Proteases can mediate degradation when samples are stored for prolonged time or samples are fractionated from starting cell or tissue preps.
Weak signal/poorly defined signal
First and foremost, the primary antibody may have low affinity for target protein. Antibody affinity may also change after denaturation of a cell/tissue sample with SDS.
1. Low antibody concentration. Increase the primary dilution.
2. Incubation times too brief.
3. Insufficient protein loaded onto gel. Load more protein.
5. Exposure of film too brief. Try multiple exposures extending from 1 minute all the way to overnight.
6. Bald Spots: bubbles between gel and membrane: bubbles create points of high resistance that lead to low transfer efficiency, be sure to remove bubbles completely when putting together the transfer sandwich.
7. Incomplete Transfer.
One of several technical errors can be the source of incomplete transfer
- Proteins not completely eluted out of gel: this often occurs with high molecular weight proteins, especially when using a transfer buffer containing methanol. One way to overcome this phenomenon is by using nitrocellulose, which does not require methanol in the transfer buffer. Adding SDS to the transfer buffer as well as using higher field strengths also improves protein elution.
- Proteins have transferred through membrane: this may occur when working with proteins of very low molecular weight. Optimizing/shortening transfer times and using a double layer of membrane usually enables retention of small proteins.
- Inappropriate transfer buffer used: the most stable and commonly used buffers are Tris-Glycine based and contain methanol.
- Impurities in the transfer buffer: this will lead to a pattern on the membrane that mirrors the holes in the transfer cassette. Fresh buffer should be prepared prior to each transfer process.