Griffin:shRNA Transfection: Difference between revisions
(→CMV) |
|||
| Line 225: | Line 225: | ||
===CMV=== | ===CMV=== | ||
CMV is a [http://en.wikipedia.org/wiki/RNA_polymerase_II RNA Polymerase II promoter]. | CMV is a [http://en.wikipedia.org/wiki/RNA_polymerase_II RNA Polymerase II promoter]. This strong promoter is active in a broad range of cell types and performs better than most pol III promoters under long term selection. However, there are specific reports that CMV driven shRNA do not function properly certain cell types, including primary CD34+ hematopoietic progentior cells. | ||
Revision as of 19:33, 13 November 2008
shRNA Transfer Vector Overview
Short hairpin RNAs (shRNAs) are typically modeled after miRNA hairpin precursors and cloned into a transfer vector that is suitable for either direct transient transfection of the shRNA, or for packaging into a lentiviral particle. Lentiviral transfer vectors are ~8-10 kb in length.
Transient shRNA Transfection
Instead of chemically synthesizing the siRNAs before introducing it in the cell, the siRNAs are made directly by the cells through an expression vector that is transiently transfected into a dividng cell. The shRNA transfer vector alone can be transiently introduced into the dividing cell where the shRNA is synthesized by cellular machinery. While transient transfection is advantageous for fast analysis of shRNA mediated effects, stable transfection ensures long-term, reproducible as well as defined shRNA effects.
Stable shRNA Transfection
For many disease models, the most desirable cell types such as immune system or primary cells are not amenable to transfection. Viral delivery of RNAi vectors is a powerful alternative to transfection for these cell types as well as for in vivo applications. Stable expression is achieved by integration of the gene of interest into the target cell's chromosome: Initially the shRNA of interest has to be introduced into the cell, subsequently into the nucleus, and finally it has to be integrated into chromosomal DNA.
Stable expression can be influenced by two factors: The transfection method used and the vector containing the shRNA of interest. The transfection method determines which cell type can be targeted for stable integration through antibiotic selection. While many lipofection reagents transfect DNA up to a certain amount into adherent cell lines, efficient delivery of DNA into difficult-to-transfect suspension cell lines or even primary cells is only possible with viral methods and nucleofection. Nucleofection is a non-viral method of introducing DNA molecules efficiently into the nucleus of dividing cells, therefore significantly increasing the chances of chromosomal integration of the transgene. The technology was pioneered by Amaxa
Vector dependent
Although there is still some debate as to the effectiveness of this approach, a regular shRNA transfer vector may be able to integrate into the genome of the target cell by antibiotic selection alone. The process may occur randomly by the cell's machinery itself, possibly via DNA repair and recombination enzymes. If this phenomenon does occur, integration into inactive heterochromatin may result in little or no shRNA expression, whereas integration into active euchromatin may allow for shRNA expression. However, random integration could also lead to silencing of the shRNA cassette. Several strategies have been developed to overcome the negative position effects of random integration: Site-specific, homologous and transposon-mediated integration strategies are used but require the expression of integration enzymes or additional sequences on the plasmid.
Lentiviral particle dependent
Lentiviral particles are highly efficient at infection and stable integration of the shRNA into a cell system. To obtain the lentiviral particle, the transfer vector that contains the shRNA cassette is already flanked by LTRs and the Psi-sequence of HIV. The LTRs are necessary to integrate the shRNA cassette into the genome of the target cell, just as the LTRs in HIV integrate the dsDNA copy of the virus into its host chromosome. The Psi-sequence acts as a signal sequence and is necessary for packaging RNA with the shRNA into pseudovirus particles. Viral proteins which make virus shells are provided in the packaging cell line (HEK 293T), but are not in context of the LTRs and Psi-sequences and so are not packaged into virions. Thus, virus particles are produced that are replication deficient. Lentiviral particles can infect both dividing and nondividing cells because their preintegration complex (virus “shell”) can get through the intact membrane of the nucleus of the target cell.
- Lentiviral systems efficiently transduce both dividing and non-dividing cells
- Study long-term gene knockdown with stable expression
- Reproducibly transduce cell populations
- Inducible or constitutive gene knockdown
Lentiviral Particle Overview
Lentiviral vectors are usually created in a transient transfection system in which a cell line (HEK293T) is co-transfected with three separate plasmid expression systems. These include the transfer vector plasmid (portions of the HIV provirus & shRNA cassette), the packaging plasmid or construct, and a plasmid with the heterologous envelop gene (ENV) of a different virus. The three plasmid components of the vector are put into a packaging cell which is then inserted into the HIV shell. Pseudoviral particles are then collected and must undergo titration to measure infectivity prior to experimental use.
Transfer Vector Plasmid
The transfer vector plasmid (~8-10 kb) contains cis-acting genetic sequences necessary for the vector to infect the target cell and for transfer of the shRNA cassette, and contains restriction sites for insertion of the shRNA. The 3’ and 5’ LTRs, the original envelop proteins, and gag sequence promoter have been removed. The shRNA transfer vector alone can be transiently introduced into the dividing cell where the shRNA is synthesized by cellular machinery.
In order to culture the transfer vector effectively and avoid recombination events, a suitable e. coli strain Invitrogen MAX Efficiency® Stbl2™ Competent Cells SKU# 10268-019, and culture temperature; 30°C expression, incubation temperature is important to optimize performance of the competent cells to produce intact lentivector.
However, there are distinct differences between E. Coli-dependent vector-based recombination events versus point mutations. Both can influence the fidelity of the lentivector, however the implications of point mutations in the construct is much more significant. Since the labor costs associated with cloning and production of the shRNA constructs are significant, be sure to invest your time in a transfer vector that is proven in literature or contains lot specific data for adequate controls from the vendor. DO NOT assume the vector you are purchasing on paper is the one you receive in the vial.
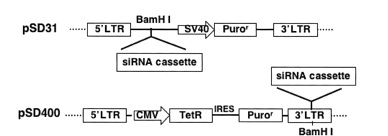
lentivector pSD31 for constitutive shRNA expression and pSD400 for conditional shRNA expression
Constitutive transfer vectors
Currently, most siRNA expression vectors are engineered to drive siRNA transcription from the polymerase III promoters U6 and H1. These promoters are particularly suited for hairpin siRNA (shRNA) expression, since they contain all of the cis-acting promoter elements upstream of the transcription initiation site and deploy a polyT transcription termination site that leads to the addition of 2-nucleotide (nt) overhangs (UU) to shRNAs, a feature that is important for siRNA function.
- Zhang J, Wang C, Ke N, Bliesath J, Chionis J, He QS, Li QX, Chatterton JE, Wong-Staal F, and Zhou D. A more efficient RNAi inducible system for tight regulation of gene expression in mammalian cells and xenograft animals. RNA. 2007 Aug;13(8):1375-83. DOI:10.1261/rna.520707 |
- Paule MR and White RJ. Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res. 2000 Mar 15;28(6):1283-98. DOI:10.1093/nar/28.6.1283 |
Inducible transfer vectors
Specific inhibition of gene expression using a stably integrated siRNA (Tiscornia et al. 2003) is not suitable for silencing genes that are involved in cell growth or survival (Zhou et al. 2006). In such cases, regulating siRNA expression in mammalian cells has become essential. Having the ability to control when and how much of a particular siRNA is expressed makes it possible to study temporal- and concentration-dependent effects on a host system and allow cells to grow prior to gene silencing by “toxic” siRNAs. Furthermore, studying a host cell's response to the presence and absence of siRNA often leads to further understanding of the target gene's function.
The inducible RNAi system uses a modified form of the regulated, tetracycline-controlled gene expression system described by Gossen and Bujard (2002) in mammalian cells for the purpose of temporal silencing target genes. This system relies on two components: a tetracycline regulatory protein (TetR), which has affinity to the tetracycline operator (TetO); and a TetO-tethered pol III promoter, whose transcription activity is blocked by binding to the regulatory TetR. In the absence of tetracycline (Tet), the TetR protein binds to the TetO sequence within the promoter and acts as a potent transcription suppressor. The siRNA expression is restored when the cell culture medium is added with either Tet or doxycycline (Dox), a Tet derivative, which binds to and causes dissociation of TetR from the pol III promoter via a TetR conformation change (Zhou et al. 2006). This system allows for observation of the loss-of-function phenotypes under noninduced and induced conditions in the same isogenic cells, excluding other potential interferences.
- Zhang J, Wang C, Ke N, Bliesath J, Chionis J, He QS, Li QX, Chatterton JE, Wong-Staal F, and Zhou D. A more efficient RNAi inducible system for tight regulation of gene expression in mammalian cells and xenograft animals. RNA. 2007 Aug;13(8):1375-83. DOI:10.1261/rna.520707 |
- Qin XF, An DS, Chen IS, and Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):183-8. DOI:10.1073/pnas.232688199 |
- Tiscornia G, Singer O, Ikawa M, and Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1844-8. DOI:10.1073/pnas.0437912100 |
- Gossen M and Bujard H. Studying gene function in eukaryotes by conditional gene inactivation. Annu Rev Genet. 2002;36:153-73. DOI:10.1146/annurev.genet.36.041002.120114 |
- Zhou D, He QS, Wang C, Zhang J, and Wong-Staal F. RNA interference and potential applications. Curr Top Med Chem. 2006;6(9):901-11. DOI:10.2174/156802606777303630 |
Envelope Gene Plasmid
The third plasmid’s envelope gene of a different virus specifies what type of cell to target and infect. Normally HIV can infect only helper T-cells because they use their gp120 protein to bind to the CD4 receptor. However, it is possible to genetically exchange the CD4 receptor-binding protein for another envelope protein that codes for different cell types. This gives the HIV lentiviral vector a broad range of possible target cells. There a few types of heterologous envelope proteins.
There are two major points to consider when choosing which coat (pseudotype). First, because VSV-G pseudotyped virus may infect human tissue, it may not be preferable to use if one is working strictly in a mouse system. Working with human infectious agents will likely require a heightened BLS lab safety level. A second point of consideration is the limitations imposed by the ecotropic coat: it is difficult to concentrate the virus due to the inherent instability of the ecotropic coat. Virus ultracentrifugation concentration is often necessary when infecting certain cell types or when generating transgenic mice, and for this reason, many choose to use VSV-G pseudotyped virus because the particles are stable to the high g-forces of ultracentrifugation. Other Polyethylene Glycol based concentration methods exist that may effectively address this concern. Even with highly concentrated virus, there is a possibility that the VSV-G or ecotropic pseudotypes may still display differing cell-type specificities for infection, an important consideration when planning an experiment. For example, in the CNS, VSV-G mediates vector entry primarily in neurons, while astrocytes appear to be better infected using other envelopes.
There are a growing number of examples of viral coat proteins for pseudotyping the virus that influences the tropism of infection in cell type in vitro, and tissue/organ type in vivo.
Envelope (viral coat) proteins






Ebola virus
(Filovirus): Allows transduction of apical surface airway epithelium. Zaire subtype EBO virus glycoproteins (GP) EboZ pseudotypes were able to efficiently transduce after apical airway epithelial application. Broad tropism for mammalian cell types.
- Powers AM, Brault AC, Shirako Y, Strauss EG, Kang W, Strauss JH, and Weaver SC. Evolutionary relationships and systematics of the alphaviruses. J Virol. 2001 Nov;75(21):10118-31. DOI:10.1128/JVI.75.21.10118-10131.2001 |
- Kobinger GP, Weiner DJ, Yu QC, and Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001 Mar;19(3):225-30. DOI:10.1038/85664 |
Lymphocytic choriomeningitis virus (LCMV)
A noncytopathic arenavirus shown to infect a broad range of different cell types. Less inflammatory with tropism for the liver.
- Beyer WR, Westphal M, Ostertag W, and von Laer D. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J Virol. 2002 Feb;76(3):1488-95. DOI:10.1128/jvi.76.3.1488-1495.2002 |
- Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD, Ratliff KL, Shen H, Barker CK, Martins I, Sharkey CM, Sanders DA, McCray PB Jr, and Davidson BL. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol. 2002 Sep;76(18):9378-88. DOI:10.1128/jvi.76.18.9378-9388.2002 |
Marburg virus
(Filovirus): Allows transduction of apical surface airway epithelium. Broad tropism for mammalian cell types.
- Sinn PL, Hickey MA, Staber PD, Dylla DE, Jeffers SA, Davidson BL, Sanders DA, and McCray PB Jr. Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor alpha. J Virol. 2003 May;77(10):5902-10. DOI:10.1128/jvi.77.10.5902-5910.2003 |
- Chan SY, Speck RF, Ma MC, and Goldsmith MA. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J Virol. 2000 May;74(10):4933-7. DOI:10.1128/jvi.74.10.4933-4937.2000 |
Mokola lyssaviruses (MK-G)
A neurotropic virus causing rabies disease. Targets neuronal cells and neural stem cells.
- Desmaris N, Bosch A, Salaün C, Petit C, Prévost MC, Tordo N, Perrin P, Schwartz O, de Rocquigny H, and Heard JM. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol Ther. 2001 Aug;4(2):149-56. DOI:10.1006/mthe.2001.0431 |
- Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD, Ratliff KL, Shen H, Barker CK, Martins I, Sharkey CM, Sanders DA, McCray PB Jr, and Davidson BL. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol. 2002 Sep;76(18):9378-88. DOI:10.1128/jvi.76.18.9378-9388.2002 |
Rabies virus
Target neuronal cells and neural stem cells.
- Desmaris N, Bosch A, Salaün C, Petit C, Prévost MC, Tordo N, Perrin P, Schwartz O, de Rocquigny H, and Heard JM. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol Ther. 2001 Aug;4(2):149-56. DOI:10.1006/mthe.2001.0431 |
- Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD, Ratliff KL, Shen H, Barker CK, Martins I, Sharkey CM, Sanders DA, McCray PB Jr, and Davidson BL. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol. 2002 Sep;76(18):9378-88. DOI:10.1128/jvi.76.18.9378-9388.2002 |
- Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000 Jun;7(11):910-3. DOI:10.1038/sj.gt.3301188 |
RD114/TR
A chimeric envelope glycoprotein made of the extracellular and transmembrane domains of the feline leukemia virus RD114 and the cytoplasmic tail of the murine leukemia virus amphotropic envelope. Transduces primary lymphocytes; RD114/TR-pseudotyped vectors showed augmented transduction of human and macaque primary blood lymphocytes and CD34+ cells.
- Di Nunzio F, Piovani B, Cosset FL, Mavilio F, and Stornaiuolo A. Transduction of human hematopoietic stem cells by lentiviral vectors pseudotyped with the RD114-TR chimeric envelope glycoprotein. Hum Gene Ther. 2007 Sep;18(9):811-20. DOI:10.1089/hum.2006.138 |
- Sandrin V, Boson B, Salmon P, Gay W, Nègre D, Le Grand R, Trono D, and Cosset FL. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002 Aug 1;100(3):823-32. DOI:10.1182/blood-2001-11-0042 |
Ross River virus (RRV; alphavirus)
Less inflammatory with tropism for the liver. RRV-pseudotyped FIV vectors (RRV/FIV) predominantly transduced the liver of recipient mice. Transduction efficiency in the liver with the RRV/FIV was ca. 20-fold higher than that achieved with the vesicular stomatitis virus G protein (VSV-G) pseudotype.
- Kahl CA, Marsh J, Fyffe J, Sanders DA, and Cornetta K. Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J Virol. 2004 Feb;78(3):1421-30. DOI:10.1128/jvi.78.3.1421-1430.2004 |
- Sharkey CM, North CL, Kuhn RJ, and Sanders DA. Ross River virus glycoprotein-pseudotyped retroviruses and stable cell lines for their production. J Virol. 2001 Mar;75(6):2653-9. DOI:10.1128/JVI.75.6.2653-2659.2001 |
- Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD, Ratliff KL, Shen H, Barker CK, Martins I, Sharkey CM, Sanders DA, McCray PB Jr, and Davidson BL. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol. 2002 Sep;76(18):9378-88. DOI:10.1128/jvi.76.18.9378-9388.2002 |
Semliki Forest virus (SFV; alphavirus)
VSV-G and RRV pseudotypes have comparable physical titers, but infectious titers with the RRV pseudotype are lower than with VSV-G. Incorporation of SFV glycoproteins into lentivirus vector is less efficient, leading to decreased physical and infectious titers. The transduction rates with VSV-G-, RRV-, and SFV-pseudotyped lentivirus vectors into adherent cell lines can be significantly increased by using a combination of Polybrene and plates coated with CH-296 recombinant fibronectin fragments.
- Kahl CA, Marsh J, Fyffe J, Sanders DA, and Cornetta K. Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J Virol. 2004 Feb;78(3):1421-30. DOI:10.1128/jvi.78.3.1421-1430.2004 |
Sindbis virus (SINV; alphavirus)
Sindbis virus encodes two transmembrane envelope proteins, E1 and E2. E2 is responsible for receptor binding; E1 is responsible for pH-dependent fusion. SINV when modified to contain the Fc-binding domain of protein A, this envelope gives a significant enhancement in specificity in combination with antibodies specific for HLA and CD4 relative to that without antibody.
- Kahl CA, Marsh J, Fyffe J, Sanders DA, and Cornetta K. Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J Virol. 2004 Feb;78(3):1421-30. DOI:10.1128/jvi.78.3.1421-1430.2004 |
- Morizono K, Bristol G, Xie YM, Kung SK, and Chen IS. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001 Sep;75(17):8016-20. DOI:10.1128/jvi.75.17.8016-8020.2001 |
Venezuelan equine encephalitis virus (VEEV; alphavirus)
Venezuelan equine encephalitis virus (VEEV) is a distant relative of SINV, RRV, and SFV and is a representative of the New World alphaviruses. It is an arbovirus that is normally maintained in a “silent” enzootic cycle between mosquitoes of Culex (Melanoconion) spp. and rodent reservoir hosts.
- Kolokoltsov AA, Weaver SC, and Davey RA. Efficient functional pseudotyping of oncoretroviral and lentiviral vectors by Venezuelan equine encephalitis virus envelope proteins. J Virol. 2005 Jan;79(2):756-63. DOI:10.1128/JVI.79.2.756-763.2005 |
Vesicular stomatitis virus G glycoprotein (VSV-G)
Highly efficient particle production. Broad tropism. Less inflammatory with in vivo tropism for the liver.
- Burns JC, Friedmann T, Driever W, Burrascano M, and Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033-7. DOI:10.1073/pnas.90.17.8033 |
- Borok Z, Harboe-Schmidt JE, Brody SL, You Y, Zhou B, Li X, Cannon PM, Kim KJ, Crandall ED, and Kasahara N. Vesicular stomatitis virus G-pseudotyped lentivirus vectors mediate efficient apical transduction of polarized quiescent primary alveolar epithelial cells. J Virol. 2001 Dec;75(23):11747-54. DOI:10.1128/JVI.75.23.11747-11754.2001 |
- Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000 Jun;7(11):910-3. DOI:10.1038/sj.gt.3301188 |
- Desmaris N, Bosch A, Salaün C, Petit C, Prévost MC, Tordo N, Perrin P, Schwartz O, de Rocquigny H, and Heard JM. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol Ther. 2001 Aug;4(2):149-56. DOI:10.1006/mthe.2001.0431 |
Packaging Plasmid
The packaging plasmid is the backbone of the virus system. In this plasmid are found the elements required for vector packaging such as structural proteins, HIV genes (except the gene env which codes for infection of T cells, or the vector would only be able to infect these cells), and the enzymes that generate vector particles. Also contained is the human cytomegalovirus (hCMV) which is responsible for the expression of the virus proteins during translation. The packaging signals and their adjacent signals are removed so the parts responsible for packaging the viral DNA have been separated from the parts that activate them. Thus, the packaging sequences will not be incorporated into the viral genome and the virus will not reproduce after it has infected the host cell.
- Amado RG and Chen IS. Lentiviral vectors--the promise of gene therapy within reach?. Science. 1999 Jul 30;285(5428):674-6. DOI:10.1126/science.285.5428.674 |
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, and Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263-7. DOI:10.1126/science.272.5259.263 |
Vector Titration
Vector titration typically involves PCR or flow cytometry depending on the presence of a fluorescent protein. For example, mouse NIH 3T3 or human HT1080 cells are infected for 4 hours with limiting dilutions of vector supernate in the presence of polybrene and analyzed for expression of fluorescent protein or detection of vector or packaging sequences by PCR. The titer represents the relative infectivity of the vector as measured on the target cell of choice and is expressed as Infectious Units (IU)/mL. Different target cells, or different infection protocols, may yield different results.
Vector supernate can be concentrated using ultrafiltration. Recoveries (65-90%) vary depending on the envelope used and stability of the viral particle. This technique does not separate empty from functional viral particles. Therefore, at very low titers, concentration may not always yield an improved titer due to the presence of inhibitory empty viral particles.
Packaging cells
Human 293 cells are usually used for packaging, since they can be transfected with efficiencies in the range of 90-100%. If the cells do not transfect well in control experiments using a non-viral GFP vector, it may be worth discarding them and trying a different lot of cells. On occasion, cells can become refractive to transfection. Consequently, use low passage cells for all transfections. Although 293 cells are adherent, they can easily detach from the plate during pipetting, or even by jostling a plate that has reached confluency. For this reason, perform the transfection when the cells are approximately 30% confluent. After 36-48 hours, the cells should reach confluency and be producing the maximum amount of virus. If the cells are fed fresh media, several harvests of virus can be made after the cells reach confluency. One final note: if human cells are used for packaging a RNA hairpin that targets a human sequence, lower viral titers may be experienced if silencing of the targeted gene is detrimental to the cell or packaging process.
shRNA Transfer Vector Promoters
RNA Polymerase III type 3 promoters (H1, 7SK, U6) are common in vector-based expression because they are permissive expressers, they do not require any additional sequence elements, and have simple termination signals. The most abundant cellular RNAs transcribed from a type III RNA pol III promoter are the U6 small nuclear RNA (which play a crucial role in the processing of premature RNA); the 7SK RNA (a negative regulator of RNA polymerase II elongation factor TEFb); and H1 RNA (a component of RNAse P).
Unlike the majority of RNA pol III promoters, type 3 RNA Pol III promoters do not require any additional sequence elements. In addition, termination of transcription by Pol III occurs at definitive tracts of 4–5 thymidines (T4–5) (Example below), which can be inserted downstream of shRNA coding sequences in order to ensure direct termination. These 2 specific properties enable the U6, 7SK, and H1 promoters to be ideal candidates for in vivo delivery systems of siRNAs where DNA templates can undergo transcription into small RNAs with structural features resembling active siRNA/miRNA.
U6
U6 small nuclear RNA (snRNA) is an essential component of the eukaryotic spliceosomes, is unique in that it is synthesized by RNA polymerase III, while all other U-snRNAs are synthesized by RNA polymerase II. U6 genes are notable for functional upstream regulatory elements which resemble RNA polymerase II regulatory sequence motifs. Tests for both potency and adverse metabolic effects upon primary cells indicate that U6 promoter may exert toxicity relative to H1 or 7SK due in part to higher activity.
U6 snRNA Class III gene initiation
1. SNAPc (SNRNA Activating Protein complex) (also termed PBP and PTF) binds to the PSE (Proximal Sequence Element) centered approximately 55 base pairs upstream of the start site of transcription. This assembly is greatly stimulated by the Pol II transcription factors Oct1 and STAF that bind to an enhancer-like DSE (Distal Sequence Element) at least 200 base pairs upstream of the start site of transcription. These factors and promoter elements are shared between Pol II and Pol III transcription of snRNA genes.
2. SNAPc acts to assemble TFIIIB at a TATA box centered 26 base pairs upstream of the start site of transcription. It is the presence of a TATA box that specifies that the snRNA gene is transcribed by Pol III rather than Pol II.
3. The TFIIIB for U6 snRNA transcription contains a smaller Brf1 paralogue, Brf2. TFIIIB is the transcription factor that assembles Pol III at the start site of transcription. Sequence conservation predicts that TFIIIB containing Brf2 also plays a role in promoter opening.
7SK
7SK is an abundant and evolutionarily conserved small nuclear RNA discovered in the 1970s. 7SK is transcribed by RNA polymerase III from one or more genes belonging to a family of interspersed repeats in the mammalian genome (Murphy et al 1984)]. The human 7SK promoter presents a strong permissivity for the nucleotide in the +1 position and recognizes a cluster of 4 or more T residues as a termination signal. The human 7SK promoter is ideal for the production of shRNAs as it can generate high amounts of shRNAs (Czauderna et al 2003, Koper-Emde et al 2004). In a series of experiments aimed to compare the strength of the human 7SK, H1 and U6 promoters, the best silencing efficiencies of various target genes was consistently obtained with a 7SK promoter (unpublished data;Invivogen).
H1
The H1 promoter drives expression of a unique gene encoding H1 RNA, the RNA component of the human RNase P. The H1 RNA gene is transcribed by RNA polymerase III into a small RNA transcript. The human H1 promoter presents the characteristics of being unusually compact. All the essential elements for transcription, that is octamer, Staf, proximal sequence element and TATA motifs, lie within 100 bp of the 5‘ flanking region. This promoter is highly permissive for the nucleotide at the +1 position which is originally an A. The termination sequence consists of a stretch of 5 thymidines. The cleavage of the transcript after the termination signal occurs after the second uridine generating a 2 nt 3‘ overhang. The H1 promoter has been successfully used in different plasmids to create siRNAs.
CMV
CMV is a RNA Polymerase II promoter. This strong promoter is active in a broad range of cell types and performs better than most pol III promoters under long term selection. However, there are specific reports that CMV driven shRNA do not function properly certain cell types, including primary CD34+ hematopoietic progentior cells.
- An DS, Qin FX, Auyeung VC, Mao SH, Kung SK, Baltimore D, and Chen IS. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther. 2006 Oct;14(4):494-504. DOI:10.1016/j.ymthe.2006.05.015 |
- Myslinski E, Amé JC, Krol A, and Carbon P. An unusually compact external promoter for RNA polymerase III transcription of the human H1RNA gene. Nucleic Acids Res. 2001 Jun 15;29(12):2502-9. DOI:10.1093/nar/29.12.2502 |
- Tiscornia G, Singer O, Ikawa M, and Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1844-8. DOI:10.1073/pnas.0437912100 |
- Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, and Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5515-20. DOI:10.1073/pnas.082117599 |
- Danzeiser DA, Urso O, and Kunkel GR. Functional characterization of elements in a human U6 small nuclear RNA gene distal control region. Mol Cell Biol. 1993 Aug;13(8):4670-8. DOI:10.1128/mcb.13.8.4670-4678.1993 |
- Koper-Emde D, Herrmann L, Sandrock B, and Benecke BJ. RNA interference by small hairpin RNAs synthesised under control of the human 7S K RNA promoter. Biol Chem. 2004 Sep;385(9):791-4. DOI:10.1515/BC.2004.103 |
- Yu JY, DeRuiter SL, and Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A. 2002 Apr 30;99(9):6047-52. DOI:10.1073/pnas.092143499 |
shRNA Transfer Vector Transfection Reagents
Transient gene silencing using RNAi is critically dependent on highly efficient delivery of the shRNA transfer vector or siRNAs into cells. The two conventional reagent types are Cationic lipid-based and Polymeric formulations. All commercial transfection reagents are proprietary formulations that are competing for market share by claiming certain advantages (ie, broad cell type compatibility, low cytotoxicity, high efficiency).
Cationic lipid
Cationic lipid transfection reagents are suitable for transfecting into a wide variety of dividing cell cultures. Commercial examples include: Lipofectamine / L2000, Dharmafect, iFect, and TransIT TKO. Cationic lipids work by forming lipsomal vesicles that house the siRNA payload and bleb their way through the living cell membrane and into the cytoplasm. The efficiency of this process must be determined in order to have confidence in the knockdown effects. There are numerous commercial sources for transfection reagents for good reason; there are numerous cell types and lipsome structure will influence transfection efficiency in the multitude of experimental cell types that exist.
Polymeric
Polymeric formulations have been developed and optimized for transfection of shRNA plasmid DNA into the nucleus of cultured eukaryotic cells by vendors such as Open Biosystems. Cationic lipids but not polyethylenimine or polylysine prevent transgene expression when complexes are injected in the nucleus (Pollard et al 1998). Polymers but not cationic lipids promote gene delivery from the cytoplasm to the nucleus and transgene expression in the nucleus is prevented by complexation with cationic lipids but not with cationic polymers.
shRNA Transfer Vector Transient Transfection Procedure
- In a six well tissue culture plate, grow cells to a 50-70% confluency in antibiotic-free normal growth medium supplemented with FBS.
NOTE: This protocol is recommended for a well from a 6 well tissue culture plate. Adjust cell and reagent amounts proportionately for wells or dishes of different sizes.
NOTE: Healthy and subconfluent cells are required for successful transfection experiments. It is recommended to ensure cell viability one day prior to transfection.
Prepare the following solutions:
NOTE: The optimal shRNA Plasmid DNA:shRNA Plasmid Transfection Reagent ratio should be determined experimentally beginning with 1 μg of shRNA Plasmid DNA and between 1.0 and 6.0 μl of shRNA Plasmid Transfection Reagent as outlined below. Once the optimal shRNA Plasmid DNA:shRNA Plasmid Transfection Reagent ratio has been identified for a given cell type, the appropriate amount of shRNA Plasmid DNA/shRNA Plasmid Transfection Reagent complex used per well should be tested to determine which amount provides the highest level of transfection efficiency. For example, if the optimal shRNA Plasmid DNA:shRNA Plasmid Transfection Reagent ratio is 1 μg:1 μl, then amounts ranging from 0.5 μg/0.5 μl to 2.0 μg/2.0 μl should be tested.
Solution A: For each transfection, dilute 10 μl of resuspended shRNA Plasmid DNA (i.e. 1 μg shRNA Plasmid DNA) into 90 μl shRNA Plasmid Transfection Medium (serum antibiotic free medium).
Solution B: For each transfection, dilute 1 - 6 μl of shRNA Plasmid Transfection Reagent with enough shRNA Plasmid Transfection Medium to bring final volume to 100 μl.
NOTE: Do not add antibiotics to the shRNA Plasmid Transfection Medium.
NOTE: Optimal results may be achieved by using siliconized microcentrifuge tubes.
NOTE: Although highly efficient in a variety of cell lines, not all shRNA Plasmid Transfection Reagents may be suitable for use with all cell lines.
- Add the shRNA Plasmid DNA solution (Solution A) directly to the dilute shRNA Plasmid Transfection Reagent (Solution B) using a pipette. Mix gently by pipetting the solution up and down and incubate the mixture 15-45 minutes at room temperature.
- Wash the cells twice with 2 ml of shRNA Transfection Medium. Aspirate the medium and proceed immediately to the next step. NOTE: Do not use PBS as the residual phosphate may compete with DNA and bind the shRNA Plasmid Transfection Reagent, thereby reducing the transfection efficiency.
NOTE: Do not use PBS as the residual phosphate may compete with DNA and bind the shRNA Plasmid Transfection Reagent, thereby reducing the transfection efficiency. For each transfection, add 0.8 ml shRNA Plasmid Transfection Medium to well.
- For each transfection, add 0.8 ml shRNA Plasmid Transfection Medium to well.
- Add the 200 μl shRNA Plasmid DNA/shRNA Plasmid Transfection Reagent Complex (Solution A + Solution B) dropwise to well, covering the entire layer.
- Gently mix by swirling the plate to ensure that the entire cell layer is immersed in solution.
- Incubate the cells 5-7 hours at 37° C in a CO2 incubator or under conditions normally used to culture the cells.
NOTE: Longer transfection times may be desirable depending on the cell line.
- Following incubation, add 1 ml of normal growth medium containing 2 times the normal serum and antibiotics concentration (2x normal growth medium).
- Incubate the cells for an additional 18-24 hours under conditions normally used to culture the cells.
Aspirate the medium and replace with fresh 1x normal growth medium.
- Assay the cells using the appropriate protocol 24-72 hours after the addition of fresh medium in the step above.
NOTE: Controls should always be included in shRNA experiments. Control shRNAs are available as 20 μg. Each encode a scrambled shRNA sequence that will not lead to the specific degradation of any known cellular mRNA.
NOTE: For Western blot analysis prepare cell lysate as follows: Wash cells once with PBS. Lyse cells in 300 μl 1x Electrophoresis Sample Buffer (sc-24945) by gently rocking the 6 well plate or by pipetting up and down. Sonicate the lysate on ice if necessary.
NOTE: For RT-PCR analysis isolate RNA using the method described by P. Chomczynski and N. Sacchi (1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162: 156-159) or a commercially available RNA isolation kit.
shRNA Controls
Negative Controls
- Untreated Cells. Untreated cells will provide a reference point for comparing all other samples.
- Empty construct, containing no shRNA insert; The empty viral particles or DNA are a useful negative control that will not activate the RNAi pathway because it does not contain an shRNA insert. It will allow for observation of cellular effects of the transduction/transfection process. Cells transduced/transfected with the empty control provide a useful reference point for comparing specific knockdown.
- Non-targeting shRNA; This non-targeting shRNA is a useful negative control that will activate RISC and the RNAi pathway, but does not target any human or mouse genes. The short hairpin sequence cotnains 5 base pair mismatches to any known human or mouse gene. This allows for examination of the effects of shRNA transduction/transfection on gene expression. Cells transduced/transfected with the non-target shRNA will also provide useful reference for interpretation of knockdown.
Positive Controls
- Positive reporter vector or lentiviral particles; This is a useful positive control for measuring transduction/transfection efficiency and optimizing shRNA delivery. The GFP Control contains a gene encoding GFP, driven by the CMV promoter. This control provides fast visual confirmation of successful transduction/transfection.
- Positive shRNA knockdown control; This control contains shRNA sequence that targets GFP expression. This shRNA control has been experimentally shown to reduce GFP expression. This control serves to quickly visualize knockdown in cells expressing GFP.
- Positive shRNA knockdown control; This control contains shRNA sequence that targets eGFP expression (GenBank Accession # pEGFP U476561). The shRNA has been experimentally shown to reduce eGFP expression by 90% in C166-GFP mouse fibroblast cells 48 hours post-transduction by mRNA transcript level. This control serves to quickly visualize knockdown in cells expressing eGFP.
Factors Influencing Successful Transfection
Concentration and purity of nucleic acids
Determine the concentration of your DNA using 260 nm absorbance. Avoid cytotoxic effects by using pure preparations of nucleic acids.
Transfection in serum-free media
The highest transfection efficiencies can be obtained if the cells are exposed to the transfection complexes in serum free conditions followed by the addition of medium containing twice the amount of normal serum to the complex medium 3–5 hrs post transfection (leaving the complexes on the cells). However, the transfection medium can be replaced with normal growth medium if high toxicity is observed.
No antibiotics in transfection medium
The presence of antibiotics can adversely affect the transfection efficiency and lead to increased toxicity levels in some cell types. It is recommended that these additives be initially excluded until optimized conditions are achieved, then these components can be added, and the cells can be monitored for any changes in the transfection results.
High protein expression levels
Some proteins when expressed at high levels can by cytotoxic; this effect can also be cell line specific.
Cell history, density, and passage number
It is very important to use healthy cells that are regularly passaged and in growth phase. The highest transfection efficiencies are achieved if cells are plated the day before. However, adequate time should be allowed to allow the cells to recover from the passaging (generally >12 hours). Plate cells at a consistent density to minimize experimental variation. If transfection efficiencies are low or reduction occurs over time, thawing a new batch of cells or using cells with a lower passage number may improve the results.
References
- Murphy S, Altruda F, Ullu E, Tripodi M, Silengo L, and Melli M. DNA sequences complementary to human 7 SK RNA show structural similarities to the short mobile elements of the mammalian genome. J Mol Biol. 1984 Aug 25;177(4):575-90. DOI:10.1016/0022-2836(84)90038-x |
- Czauderna F, Santel A, Hinz M, Fechtner M, Durieux B, Fisch G, Leenders F, Arnold W, Giese K, Klippel A, and Kaufmann J. Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res. 2003 Nov 1;31(21):e127. DOI:10.1093/nar/gng127 |
- Koper-Emde D, Herrmann L, Sandrock B, and Benecke BJ. RNA interference by small hairpin RNAs synthesised under control of the human 7S K RNA promoter. Biol Chem. 2004 Sep;385(9):791-4. DOI:10.1515/BC.2004.103 |
- Whither RNAi?. Nat Cell Biol. 2003 Jun;5(6):489-90. DOI:10.1038/ncb0603-490 |
- Pollard H, Remy JS, Loussouarn G, Demolombe S, Behr JP, and Escande D. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J Biol Chem. 1998 Mar 27;273(13):7507-11. DOI:10.1074/jbc.273.13.7507 |