Griffin:siRNA transfection

RNA interference is an epigenetic system within living cells that modulates global gene expression fluctuations.
The native RNAi pathway involves a grooming process where long double-stranded RNA (dsRNA) molecules are refined into double stranded RNA of ~20 nucleotides (first step in the figure to the right). However, the ~20 nucleotide double strand RNA (a substrate for Dicer/R2D2) is now a conventional research product known as siRNA. These ~20 nucleotide siRNA inside a cell is catalytic unwound and one of the two strands, known as the guide strand or antisense strand, is then loaded into the RNA-induced silencing complex (RISC). Experimentally, any mRNA that shares 100% sense compliment sequence to the 'loaded' RISC complex, will bind and undergo nuclease-dependent degradation. As mRNA levels go down, so can protein expression. This is an attractive technique for scientists who are seeking to better understand the importance of the target gene function by 'knocking down' gene expression in a cell system.
siRNA-dependent RNAi is a user friendly technique that allows the scientist to titrate moles siRNA/ number of cells, with a minimum number of steps toward achieving mRNA knockdown.
siRNA calculation and reconstitution
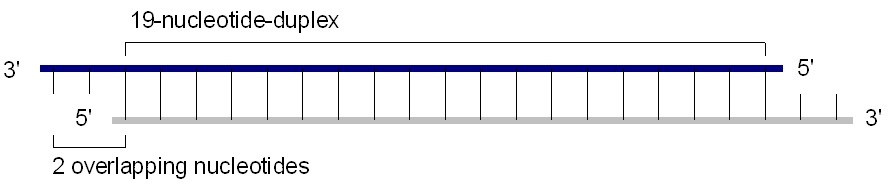
Chemically synthesized 21-25 nucleotide siRNA are suitable for transfection into dividing cells. A standard siRNA molecule is a chemically synthesized, 19 base pair double stranded RNA molecule with 2 base pair overhangs on the 3' ends (total of 21 base pair). Calculating the molecular weight of a single base pair based on chemical formula (at pH 7) gives ~615.4 Daltons/base pair on average+2 Na+ gives ~46 extra Daltons per base pair, or 661.4 Da/base pair total (average of GC & AT).
- Molarity of siRNA vialed: 10 uM ( uM/L )
- Volume after reconstitution: 330 uL
- Approximate mass of a mole of siRNA: 13800 g/mol ( 21nt X 660 g/base pair)
- Determine total mols per vial 10 um/L X 330 uL = 3.3 nm
- Convert mols to grams 3.3 nm X 13800 g/mol = 45.5 ug
- determine solution conc. 45.5 ug/ 330 uL = 0.138 ug/uL
After applying the right conversion factors (1Da = g/mol) = 0.14 ug/uL of siRNA @ 10 uM
Dissolve lyophilized siRNA duplex in RNase-free dH2O to 10 uM. Allow siRNAs to dissolve for 30 minutes at 4°C with gentle vortexing. In order to dissolve the pellet complete, it may be necessary to incubate the siRNA at 37° C for 45 minutes.
RNA Purity
Concentration of nucleic acid can be determined using the Beer-Lambert law, which predicts a linear change in absorbance with concentration.
- RNA absorption maximum =260 nm
- Pure RNA A260/A280 =2.1
- A value of 1.8-2.0 indicates that the RNA is pure.
- Adjusting pH of water solvent for spectroscopy from ~5.4-7.5/8.5 increases RNA A260/A280 ratios from approximately 1.5 to 2.0.
Cationic lipid-based transfection
Cationic lipid-based transfection reagents are suitable for transfecting into dividing cell cultures. Commercial examples include: Lipofectamine / L2000, Dharmafect, iFect, and TransIT TKO.
All of these reagents are proprietary formulations that operate by forming lipsomal vesicles that house the siRNA payload and the slight + charge of the liposome is drawn to the cell membrane and bleb their way through the living cell membrane and into the cytoplasm.
The efficiency of this process is cell type-dependent and must be determined in order to have confidence that the siRNA is getting into the cell.
There are numerous commercial sources for transfection reagents for good reason; there are numerous cell types and lipsome structure will influence transfection efficiency in the multitude of experimental cell types that exist.
In summary, the efficiency of a transfection reagent is a cell type-dependent event. Not all cells are receptive to transfection and for this reason, before any experiments are done, the researcher must determine transfection efficiency.
Verify transfection efficiency

The efficiency of transfection may depend on the cell type, but also on the passage number and the confluency of the cells. The time and the manner of formation of siRNA-liposome complexes (e.g. inversion versus vortexing) are also critical.
Verify the transfection efficiency with the use of a fluorescent dye-labeled siRNA; Control siRNA (FITC Conjugate)-A: sc-36869 (FITC, CY5, etc.). A flow cytometer can be used to measure cell transfection efficiency. The transfection efficiency must be measured 5-7 hours after cells are first exposed to the transfection reagent. This is important as the siRNA reagent complex with the siRNA will maximal enter the cell during this time frame. After 5-7 hours, the cells could begin to degrade or exotise the control siRNA-FITC conjugate and this will.
When transfecting adherent cells, 5-7 hours after transfection, wash the slide then fix and mount the cells prior to visualization under a fluorescent scope.
1) In a six well tissue culture plate, seed 2e5 cells per well in 2 ml antibiotic-free normal growth medium supplemented with FBS.
2) Incubate the cells at 37° C in a CO2 incubator until the cells are 50 - 80% confluent. This will usually take 18-24 hours.
NOTE: Healthy and subconfluent cells are required for successful transfection experiments. It is recommended to ensure cell viability one day prior to transfection.
3) Prepare the following solutions:
Solution A: For each transfection, dilute 20-80 pmols siRNA into 100 µl siRNA Transfection Medium (serum/antibiotic-free medium). Solution B: For each transfection, dilute 2-8 µl of siRNA Transfection Reagent (brought to room temperature) into 100 µl siRNA Transfection Medium. Peak activity should be at about 6 µl siRNA Transfection Reagent.
4) Add the siRNA duplex solution (Solution A) directly to the dilute Transfection Reagent (Solution B) using a pipette. Mix gently by pipetting the solution up and down and incubate the mixture 15–45 minutes at room temperature.
5) Wash the cells once with 2 ml of serum/antibiotic-free medium and aspirate the medium and proceed immediately to the next step.
6) For each transfection, add 0.8 ml serum/antibiotic-free medium to each tube containing the siRNA Transfection Reagent mixture (Solution A + Solution B). MIx gently and overlay the mixture onto the washed cells. Total volume per well should be 1 mL.
NOTE: 20-80 pmols of siRNA in total volume of 1 ml (good for 1 well in a 6 well plate)= 20-80 nanomolar
7) Incubate the cells 5–7 hours at 37° C in a CO2 incubator.
8) Measure transfection efficiency: The transfection efficiency must be measured 5-7 hours after cells are first exposed to the transfection reagent.
Once the cell type is determined to be suitable for the chosen transfection reagent by measuring transfection efficiency, the RNAi procedure is ready to try.
siRNA transfection Procedure 6 well
Optimal conditions for transfection will vary. Amount of siRNA used for transfection may vary for each target protein and should be determined experimentally. Titrate the cell density parameter by trying different confluency of cells since different cell types can achieve an optimized knockdown at different cell densities. Cell density is an important variable. Different amounts of siRNA should be used (15, 30, 60, 90 nM) to determine what the minimal concentration necessary is to achieve efficient gene silencing. The amount of transfection reagent used per transfection is also an important variable and using more or less can improve transfection effiicency and cell viability.
1) In a six well tissue culture plate, seed 2 x 105 cells per well in 2 ml antibiotic-free normal growth medium supplemented with FBS.
2) Incubate the cells at 37° C in a CO2 incubator until the cells are 50 - 80% confluent. This will usually take 18-24 hours.
NOTE: Healthy and subconfluent cells are required for successful transfection experiments. It is recommended to ensure cell viability one day prior to transfection.
3) Prepare the following solutions:
Solution A: For each transfection, dilute 20-80 pmols siRNA into 100 µl siRNA Transfection Medium (serum/antibiotic-free medium). Solution B: For each transfection, dilute 2-8 µl of siRNA Transfection Reagent (brought to room temperature) into 100 µl siRNA Transfection Medium. Peak activity should be at about 6 µl siRNA Transfection Reagent.
4) Add the siRNA duplex solution (Solution A) directly to the dilute Transfection Reagent (Solution B) using a pipette. Mix gently by pipetting the solution up and down and incubate the mixture 15–45 minutes at room temperature.
5) Wash the cells once with 2 ml of serum/antibiotic-free medium and aspirate the medium and proceed immediately to the next step.
6) For each transfection, add 0.8 ml serum/antibiotic-free medium to each tube containing the siRNA Transfection Reagent mixture (Solution A + Solution B). MIx gently and overlay the mixture onto the washed cells. Total volume per well should be 1 mL.
NOTE: 20-80 pmols of siRNA in total volume of 1 ml (good for 1 well in a 6 well plate)= 20-80 nanomolar
7) Incubate the cells 5–7 hours at 37° C in a CO2 incubator.
8) Add 1 ml of normal growth medium containing 2 times the normal serum and antibiotics concentration (2x normal growth medium) without removing the transfection mixture. If toxicity is a problem, remove the transfection mixture and replace with 1x normal growth medium.
9) Incubate the cells for an additional 18–24 hours.
10) Aspirate the medium and replace with fresh 1x normal growth medium.
11) Assay the cells using the appropriate protocol 24–72 hours after the addition of fresh medium in the step above.
NOTE: The 12 well plate has a smaller well volume than a 6 well, so consider using less volume per well on a 12 well. siRNA concentration can affect cell viability. Experimental range that is common for siRNA is 1-100 nM. The issue of off-target effects must be addressed by running a control (non-targeting) siRNA. The control siRNA should be run in parallel at the same concentration as a way to ensure the result is authentic depending on what they are measuring (mRNA, protein, function).
Cells are incubated for 16 hours at 37°C and 5%CO2. Check for alteration in cell morphology or cytotoxic effects after siRNA transfection. This is a key step to visualize the cells and determine if they are healthy or if cytotoxicity is an issue. Cytotoxicity can be minimized by replacing the transfection solution with normal growth medium as early as 7 hours into transfection. In order to monitor gene silencing at the protein level proteins can be extracted after 24, 48 and 72 hours and measured by immunoblot. Quantitative RT-PCR is an alternative method to measure mRNA level.
siRNA transfection Procedure 24 well
- Seed adherent cells 1 day prior to transfection in 24-well plates using 500 µl of DMEM tissue culture medium supplemented with 10% FBS, without antibiotics.
- For one well of a 24-well plate, use 0.84 µg siRNA duplex (60 pmole in 3 µl annealing buffer).
- Mix 3 µl of 20 µM siRNA duplex (60 picomols) with 50 µl of Opti-MEM. In another tube, mix 3 µl of OLIGOFECTAMINE Reagent (or 3 to 3.5 µl Transit TKO) with 12 µl of Opti-MEM, incubate 7 to 10 min at room temperature.
- Combine the solutions and gently mix by inversion. Do not vortex. Incubate another 20 to 25 min at room temperature; the solution turns turbid.
- Then add 32 µl of fresh Opti-MEM to obtain a final solution volume of 100 µl. (The addition of 32 µl Opti-MEM is optional and serves only to adjust the total volume of cell culture medium to 600 µl after transfection.)
- Add the 100 µl of siRNA-OLIGOFECTAMINE to cultured cells (~40 to 50% confluent). Total volume is 600ul/well (60 pmol/600 ul =100 nM siRNA).
- After 5-7 hours, add normal growth medium containing normal serum and antibiotics concentration without removing the transfection mixture. If toxicity is a problem, remove the transfection mixture and replace with 1x normal growth medium.
- Incubate the cells for an additional 18–24 hours.
- Aspirate the medium and replace with fresh 1x normal growth medium.
- Assay the cells using the appropriate protocol 24–72 hours post transfection.
Working Cell Density Parameters
Suspension Cells
Suspension cells require slow speed centrifugation (2500xg) to collect intact cell pellets from cuture flasks, then counting the cells with a hemocytometer and then seeding the wells accordingly by cell count into transfection medium. Suspension cells can proceed directly to the siRNA experiment rather than adherent cells, that require seeding the cells and waiting for adherence.
Sorting Dead Suspension Cells
Dead Cells are generally lighter than live cells. Resuspend the cells in 15 or 50 ml conical centrifuge tubes and allow the cells to settle 2-5 minutes. Most live cells will sediment to the bottom, leaving a mixture of Live/Dead cells floating in the supernatant. Aspirate supernatant and discard. This will leave the live cells ready for resuspension into the culture flask. Some live cells will be lost in this process.
Six well plates (2x10e5 cells per well)
Endothelial cells were seeded onto six well plates (2x10e5 cells per well) and incubated under normal growth conditions. The confluency of the transfected culture was about 80% at the time of transfection.
20-30% confluency
Another condition reported to be successful was in a 12-well format at relatively low density 20-30% confluency of transformed mouse adherent cell; plated 4x10e4 cells per well 40 hours before transfection.
Reverse transfection of adherent cells
Trypsinize cells and count the exact amounts per well, then perform the transfection as the cells are re-attaching to the plates. The advantage of this approach is knowing the cell count at the time of transfection and there may be a greater efficiency of uptake as the cells are re-attaching to the plates.
Analysis
Western blot and RT-PCR are good methods for knockdown analysis.
Knockdown Kinetics
mRNA levels vary depending on the target protein. There are many factors that play a role on this. To name a few: target protein half life, constitutive protein expression versus transient protein expression, number of cells transfected (i.e., confluency), amount of siRNA used for transfection, transfection efficiency, siRNA sequence selection, etc. To simplify the process you can use a standard time of 48 hours post transfection to analyze knockdown.
Once knockdown is achieved, it will remain for as long as there is available siRNA inside the cells. Typically it will last for about 72 hours after transfection. For a longer knockdown, and for proteins with long half lives, a second or multiple siRNA transfections are recommended. If you do this, please make sure your control cells go through the same manipulation as your transfected cells, to subtract non-siRNA side effects.
When working on a protein that is critical for the cell, it is possible to observe unhealthy cells or high cell death after the siRNA transfection. In this case, it is recommended to check for knockdown sooner, or to decrease the amount of siRNA used to decrease the knockdown effect.
Cell adhesion internal control
Eg-5 siRNA is a very useful control since it is critical for cell adhesion. For instance, validated expression knockdown 24 hours after transfection, which peaks at 48 hours, cells will remain viable, yet lose adherent phenotype. Eg-5 transfection causes loss of adherence of the cell.
siRNA Stability
Run the control experiment: 1 pmol (17 ng) native 2% agarose gel in the presence of ethidium bromide for visualization should see robust ~21 nt signal under UV.
siRNA Controls
Mismatch or scrambled siRNAs
These are often of somewhat limited value. A scrambled sequence is too unrelated to the 'active' probe to function as a truly informative control. Occasionally, a one- or two-base-pair change in the middle of the antisense can be a useful negative control if the siRNA effect is clearly ablated. However, such a change may have unanticipated effects by converting an siRNA into a miRNA (micro RNA) that inhibits translation through a pathway closely related to siRNA.
Basic controls
siRNAs exert their effects through a growing number of surprisingly diverse mechanisms in addition to selective degradation of the targeted mRNA, such as specific effects at the chromatin level. Currently, siRNAs, unlike long double-stranded RNA (dsRNA), are not thought to trigger general translational attenuation through the interferon response. However, this also remains a hotly debated possibility for some short siRNAs, or at least mixed populations retaining some longer dsRNA species. In addition, the closely related miRNAs do function through target-specific translational attenuation. Thus, it is important to show reduction of expression at the mRNA and protein level, as well as a functional readout where available. If both message and protein are ablated, the response is 'classical' RNAi. In contrast, if only the protein is reduced, the chances are that a miRNA-related translational mechanism is at work. It should be noted that although this set of controls should be regarded as best practice, the functional control listed below renders analysis of the mRNA non-essential. Additional useful controls are available for unintentional activation of global translational repression through the interferon response (commercial assay kits or expression of unrelated proteins). Although centromeric or other emergent chromatin effects are harder to generate controls for, global gene expression may function as a control for any non-specific effects on gene expression.
Quantitative controls
Titration of the siRNA is strongly encouraged. RNAi is often extremely effective already at minimal concentrations, and titration to the lowest possible levels reduces the chance of side effects as well as providing a graded readout of the effect. This is especially important because the RNAi machinery (the RISC complex in particular) is saturable, at least in some settings. Again, the rescue control outlined below is especially important when high levels of siRNA must be used. Protein levels should be assessed with quantitative techniques, such as quantitative western blotting, to allow for an accurate estimate of the level of reduction.
Functional controls
The ultimate control for any RNAi experiment remains rescue by expression of the target-gene in a form refractory to siRNA (ideally within the physiological range). This can often be achieved by utilizing one or more silent third-codon point mutations within the targeted region, although controls for fortuitous miRNA effects are desirable. Translational effects can be avoided by utilizing siRNAs targeted against 3'-untranslated regions (UTRs), which are non-essential for rescue expression from a plasmid. The use of recently developed vector-based RNAi systems will alleviate some of the technical hurdles of rescue expression.
Multiplicity controls
A good way to enhance confidence in RNAi data is to demonstrate a similar effect with two or more siRNAs targeted to different sites in the message under study. Alternatively, the RNAi approach is usefully supplemented by alternative methods, such as those described above.
RNase-free H2O
- 0.1% DEPC in distilled H2O. Mix and leave at room temperature for 1 hour.
- Autoclave
- Cool to room temperature prior to use.
0.1% DEPC inactivates RNase contamination from common environmental sources and laboratory procedures. Autoclaving alone will inactivate a substantial amount of RNase as well.
Autoclaving inactivates DEPC by causing hydrolysis of diethylpyrocarbonate to yield CO2 and EtOH. DEPC has a half-life of ~30 minutes in water; 0.1% DEPC solution autoclaved for 15 minutes/liter can be assumed to be DEPC-free.
TE Buffer
10X TE Buffer (siRNA dilution buffer)
- 10 mM Tris, bring to pH 7.5 with HCl
- 1 mM EDTA
- RNase free water (DEPC water)
pH and salt are a requirement for stable, double stranded oligonucleotides; to reconstitute the siRNA in just water, the complementary strands most likely will separate, rendering single stranded oligonucleotides.
The EDTA is added to prevent degradation of the double stranded oligos by DNAses. The EDTA in TE chelates Mg2+ and other divalent metals ions necessary for most causes of DNA and RNA degradation, suppressing these processes. Each EDTA molecule chelates one Mg2+ ion.
DNA is stored at pH 8 to reduce depurination, which is acid catalyzed, while RNA is stored at a slightly lower pH (7.5) because degradation of RNA is base-catalyzed. Most downstream reactions will not be influenced by the slightly different pH storage conditions.
Troubleshooting
cell type, MOI, steric hindrance between mRNA & RISC/antisense sequence, artifact effects, inflammatory response, and miRNA displacement are all possible explanations for inconclusive knockdown
TECHNICAL SERVICE GUIDE: siRNA
Catalog # Lot #
Background Info
- What are the experimental results?
- Describe how gene knockdown is measured?
- How was the RNA reconstituted?
NOTE: siRNA ships lyophilized along with RNase free water with instructions to reconstitute with 330 ul of H2O to make 10 uM solution. Having the correct molarity of the solution is critical.
- Molarity of siRNA vialed: 10 uM ( uM/L )
- Volume after reconstitution: 330 uL
- Mass of 1 mole of siRNA: 13800 g/mol ( 21nt X 660 g/base pair)
- Total mols per vial: 10 um/L X 330 uL = 3.3 nm
- Total grams per vial: 3.3 nm X 13800 g/mol = 45.5 ug
- Solution concentration: 45.5 ug/ 330 uL = 0.138 ug/uL
- Did this same vial or other lot of siRNA work in the past?
NOTE: If the siRNA same cat# has worked in the past, and now does not work, this may suggest RNase contamination. There are ways to determine this by running 1 pmol (17 ng) siRNA in a native 2% agarose gel, however replacing the vial is a straightforward solution.
Transfection Efficiency
- Describe the cell type for this experiment?
- What transfection reagent is used for the siRNA tranfection?
NOTE: Cationic lipid based transfection reagents (ie Lipofectimine, L2000, Transit TKO, Oligofect, Dharmafect, sc-29528) are each one a unique formula, that certain cell types will respond better to certain cationic lipid (positive charge lipophilic) reagents. For this reason, measuring transfection efficiency is necessary.
- How was transfection efficiency measured?
NOTE: The researcher may have an existing transfection reagent that works on their cells in other experiments (ie cDNA studies), and this is a good indicator to try the same reagent and measure transfection efficiency.
- What time point was transfection uptake of FITC-siRNA measured?
NOTE: Measuring transfection efficiency with sc-36869 will validate that liposome-dependent siRNA entry into the cells is taking place efficiently. It is important to measure transfection efficiency 5-7 hours post transfection since this is when the optimum time point where most transfection takes place. Common methods are IF or Flow cytomtetry.
Cell Confluency
- Adherent cell (grows on the surface of the plate): What is the cell confluency at time of transfection?
- Suspension cell (ie leukocytes/lymphocytes, grow in the media) : How many cell count # used to seed the well?
NOTE: A hemocytometer (cell counter) is common for counting cells for seeding into multiwell plates (6, 12, 24 well); originally designed for performing blood cell counts. Cell density is an important parameter for knockdown. Optimum cell density will vary and typically falls between 30-80%.
NOTE: Setting up a 6 or 12 well experiment and trying a range of cell confluencies (30, 50, 70%), will reveal an optimal cell density where knockdown is optimal with minimal cell death. Effective confluence can range from 30-80%.
siRNA Concentration
- What nanomolar concentration(s) of siRNA are tested?
NOTE: Setting up a 6 or 12 well experiment and trying a range of cell confluencies (30, 50, 70%) & a range of siRNA concentrations (30, 60, 90 nM) will reveal an optimal convergence of cell density and concentration of siRNA where knockdown is optimal with minimal cytotoxicity (cell death).
- What time points is RNAi measured?
NOTE: 48 hours post transfection is a relevant singular point. Measuring knockdown for a few time points in the 24-72 hour window may indicate the frame when RNAi is most optimal. Titrating the siRNA concentration (30-90 nM) for the cells will indicate the best amount to see an effect.
Measuring Knockdown
- How is RNAi measured? Western blot - IF - qPCR - other
NOTE: For WB, titrating the antibody may reveal subtle changes in knockdown. For IF, running secondary controls may indicate nonspecific fluorescence mistaken for signal.
- Quantitative RT-PCR, which primers were used and what type of system?
NOTE: With appropriate internal controls (GAPDH, DNA contamination control), qPCR can be very reliable in determining translation initiation arrest.
References
Santa Cruz Biotechnology Protocols
Design
The methodology for designing siRNA is well established in primary literature from ~2002-2005; thermodynamics, filters, motif parameters. Rational design is based on the substrate characteristics of miRNA, however does not predict steric effects for antisense recognition of the transcript, hence the attractiveness of pools.
siRNA Design Scoring
- Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, and Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004 Mar;22(3):326-30. DOI:10.1038/nbt936 |
- Ui-Tei K, Naito Y, Takahashi F, Haraguchi T, Ohki-Hamazaki H, Juni A, Ueda R, and Saigo K. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004;32(3):936-48. DOI:10.1093/nar/gkh247 |
- Amarzguioui M and Prydz H. An algorithm for selection of functional siRNA sequences. Biochem Biophys Res Commun. 2004 Apr 16;316(4):1050-8. DOI:10.1016/j.bbrc.2004.02.157 |
- Jagla B, Aulner N, Kelly PD, Song D, Volchuk A, Zatorski A, Shum D, Mayer T, De Angelis DA, Ouerfelli O, Rutishauser U, and Rothman JE. Sequence characteristics of functional siRNAs. RNA. 2005 Jun;11(6):864-72. DOI:10.1261/rna.7275905 |
- Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, and Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005 Feb;23(2):222-6. DOI:10.1038/nbt1051 |
Immunostimulatory motifs
5'-UGUGU-3', 5'-GUCCUUCAA-3'
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, and MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005 Apr;23(4):457-62. DOI:10.1038/nbt1081 |
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, and Hartmann G. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005 Mar;11(3):263-70. DOI:10.1038/nm1191 |
Seed region sequence complimentarity
- Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y, Baskerville S, Maksimova E, Robinson K, Karpilow J, Marshall WS, and Khvorova A. 3' UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods. 2006 Mar;3(3):199-204. DOI:10.1038/nmeth854 |
- Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, and Linsley PS. Widespread siRNA "off-target" transcript silencing mediated by seed region sequence complementarity. RNA. 2006 Jul;12(7):1179-87. DOI:10.1261/rna.25706 |
- Anderson EM, Birmingham A, Baskerville S, Reynolds A, Maksimova E, Leake D, Fedorov Y, Karpilow J, and Khvorova A. Experimental validation of the importance of seed complement frequency to siRNA specificity. RNA. 2008 May;14(5):853-61. DOI:10.1261/rna.704708 |
- Ui-Tei K, Naito Y, Nishi K, Juni A, and Saigo K. Thermodynamic stability and Watson-Crick base pairing in the seed duplex are major determinants of the efficiency of the siRNA-based off-target effect. Nucleic Acids Res. 2008 Dec;36(22):7100-9. DOI:10.1093/nar/gkn902 |
Off-target Effects
- Khan AA, Betel D, Miller ML, Sander C, Leslie CS, and Marks DS. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat Biotechnol. 2009 Jun;27(6):549-55. DOI:10.1038/nbt.1543 |
- Kenworthy R, Lambert D, Yang F, Wang N, Chen Z, Zhu H, Zhu F, Liu C, Li K, and Tang H. Short-hairpin RNAs delivered by lentiviral vector transduction trigger RIG-I-mediated IFN activation. Nucleic Acids Res. 2009 Oct;37(19):6587-99. DOI:10.1093/nar/gkp714 |
- van Dongen S, Abreu-Goodger C, and Enright AJ. Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods. 2008 Dec;5(12):1023-5. DOI:10.1038/nmeth.1267 |
- Tschuch C, Schulz A, Pscherer A, Werft W, Benner A, Hotz-Wagenblatt A, Barrionuevo LS, Lichter P, and Mertens D. Off-target effects of siRNA specific for GFP. BMC Mol Biol. 2008 Jun 24;9:60. DOI:10.1186/1471-2199-9-60 |