Hartman:YeastXtract: Difference between revisions
No edit summary |
No edit summary |
||
| Line 107: | Line 107: | ||
== Credits == | == Credits == | ||
YeastXtract has been developed and is maintained by the Hartman Lab, University of Alabama at Birmingham. Current version of YeastXtract was implemented by [http:// | YeastXtract has been developed and is maintained by the Hartman Lab, University of Alabama at Birmingham. Current version of YeastXtract was implemented by [http://people.pcbi.upenn.edu/~najaf/ Najaf Shah] of the University of Alabama at Birmingham and algorithms were developed by Richard Laws and Lue Ping Zhao of the Fred Hutchinson Cancer Research Center. | ||
Latest revision as of 18:45, 21 February 2011
<html> <META name="verify-v1" content="Zbreeh7OAkzkm2eZDHIqUL7wF1Iv35gn/j6gbjtLRFw=" /> </html>
Hartman Lab
University of Alabama School of Medicine
Home
People
Research
Publications
Software
Links
 http://creativecommons.org/images/public/somerights20.png This work is licensed by Hartman Lab, University of Alabama at Birmingham under a Creative Commons Attribution-NonCommercial-ShareAlike 2.5 License. For questions and comments, please contact John_Hartman SummaryClick here to launch YeastXtract (Java Webstart). Click here to download the stand-alone version and for sample images.
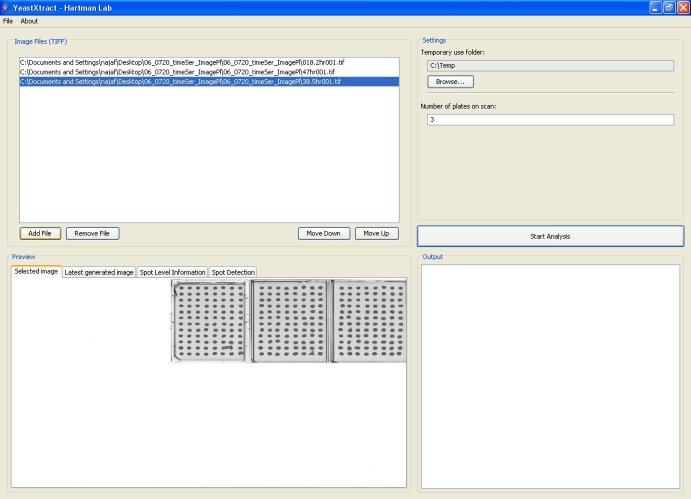
Paper describing this method: N.A. Shah, R.J. Laws, B. Wardman, L.P. Zhao and J.L. Hartman IV. Accurate, precise modeling of cell proliferation kinetics from time-lapse imaging and automated image analysis of agar yeast culture arrays. BMC Systems Biology 2007, 1:3. Paper IntroductionYeastXtract (YX) is a software application for analyzing images of yeast cell arrays and extracting high-resolution growth information. YX enables automated, high-throughput quantification of growth of microbial spot cultures with increased precision and accuracy. YX was originally developed from modifications to SignalViewer [1,2], an application for analyzing microarray images. SignalViewer was developed in Matlab and is used for alignment, spot detection, and signal extraction of spotted arrays. To promote the use of YeastXtract, we have implemented it in Java for community use. The program has additional features and intuitive user interfaces and can be used as a software module for development of automated systems for High Throughput Imaging of Cell Arrays (ISHICA). YeastXtract is available at http://openwetware.org/wiki/Hartman_Lab and can be used on any operating system that has the Java 1.5 platform installed (Java 1.5 is freely available for Microsoft Windows, Mac OS X, Linux, and UNIX operating systems). All of YeastXtract's functions can be accessed from a single screen. Users select a sequence of images by the 'Add Images' button, specify a temporary use folder via the 'Browse' button and begin analysis by clicking on the 'Start Analysis' button. After analysis is complete, the intensities and areas of culture spots are displayed in array-format in the output pane. The 'Spot Level Information' tab provides an interface for viewing time-lapse images and growth plots for individual cultures. The 'Spot Detection' tab displays images of individual plates with culture spots circumscribed by ellipses and hence enables the user to quickly assess accuracy of spot detection. Sample images, screenshots of the application, and detailed instructions are available at the website. The program and the underlying source code can be downloaded free of cost and used under the Creative Commons Attribution-ShareAlike 2.5 license. The software has a modular design to facilitate modification and development of related imaging imaging applications. YeastXtract takes as its input a time series of images of 8 x 12 cell arrays. Over time, the yeast cells grow and the culture spots become darker. YeastXtract analyzes the time-lapse images and outputs enumerated data for calculating cell proliferation phenotypes for each time series of data for a culture. To make the imaging process more efficient, plates are scanned together in groups of ten. The imaging rate is around 250 - 300 plates per hour by this manual method. System requirementsThe basic system requirements to run YeastXtract are:
InstallationInstallation via Java WebStartThe easiest way to use YeastXtract is to not install it. For most system configurations, YeastXtract can be launched by clicking on the 'Launch YeastXtract' link at the top of this page. Users are advised to try this method of running YeastXtract first. Once you click on the link, a security dialog box will ask you whether or not you want to run the application. After you click the 'Run' button, Java Webstart will automatically download and install YeastXtract and all of its associated modules; this may take several minutes, depending on the speed of your network connection. If all goes well, a screen similar to the screenshot shown below should come up. If this does not work or if you get an error message such as 'Cache viewer has unexpectedly quit,' you can try the stand-alone installer described in the next section. Installation via stand-alone installerDue to problems with Java Webstart, a minority of users might not be able to launch YeastXtract directly. Such users should still be able to run YeastXtract by downloading and installing a stand-alone version:
ProblemsYeastXtract has been tested on Windows XP, Mac OS X, and several flavors of Linux and has been found to work flawlessly on systems that meet the above-stated requirements. If for some reason you are having problems installing or running YeastXtract, please do not hesitate to contact us. We will work with you to get the program running on your machine and also fix any compatibility issues that you may uncover.
Image AnalysisQuick startTo analyze a set of images with YeastXtract, follow these steps:
Output formatWhen image analysis is complete, YeastXtract will display some text in the 'Output' box. You will notice that YeastXtract conveniently displays information in the same 8 X 12 format used in the actual plates.
To export this data, click and drag inside the 'Output' box to highlight all of its contents. Press 'Ctrl-C' and then open your favorite text editor or Microsoft Excel and press 'Ctrl-V' to paste. Spot detectionBefore looking at the data more closely, you will probably want to make some quick assessments about the accuracy of YeastXtract. To do so, click on the 'Spot Detection' tab in the main window. Next, choose a plate number from the drop-down list. Note that you cannot choose a plate that you did not analyze. The 'Preview' pane should something like the following: As shown in the above figure, YeastXtract displays an image of the specified plate and overlays ellipses calculated during its spot detection phase. If your images were in the correct format, you should see ellipses tightly circumscribing the culture spots. To get more information about a culture spot, simply click on it! Culture spotsIf you are considering using YeastXtract, you are probably most interested in getting the numerical information from the 'Output' box. However, it is often useful to look at the actual images to perform quick checks, etc. YeastXtract enables you to do just that via its 'Spot Level Information' pane. Time-lapse images of culture spots
Growth curves
References
CreditsYeastXtract has been developed and is maintained by the Hartman Lab, University of Alabama at Birmingham. Current version of YeastXtract was implemented by Najaf Shah of the University of Alabama at Birmingham and algorithms were developed by Richard Laws and Lue Ping Zhao of the Fred Hutchinson Cancer Research Center.
Note: This list is not exhaustive.
|
Hartman Lab, Division of Translational Medicine, Department of Genetics, University of Alabama at Birmingham (UAB).
Template design by IMPRS
<html>
<!-- Start of StatCounter Code -->
<script type="text/javascript" language="javascript">
var sc_project=545269;
var sc_partition=3;
var sc_invisible=1;
</script>
<script type="text/javascript" language="javascript" src="http://www.statcounter.com/counter/counter.js"></script><noscript><a href="http://www.statcounter.com/" target="_blank"><img src="http://c4.statcounter.com/counter.php?sc_project=545269&java=0&invisible=1" alt="free hit counter" border="0"> </noscript> <!-- End of StatCounter Code --> </html>