Hartman Behavioral Neuroscience Lab:Protocols: Difference between revisions
No edit summary |
|||
| (22 intermediate revisions by 4 users not shown) | |||
| Line 3: | Line 3: | ||
==Ethovision== | ==Ethovision== | ||
=== | ===Morris Water Maze Setup Instructions=== | ||
*Go to Start → Ethovision | *Go to Start → Ethovision | ||
*File → new workspace → Name file → click Save | *File → new workspace → Name file → click Save | ||
| Line 43: | Line 43: | ||
<br> | <br> | ||
===Ethovision Data Extraction Instructions=== | |||
====Cumulative Distance to Platform==== | |||
*go to ADD PARAMETER > DISTANCE TO ZONE BORDER... you'll notice that it defaults to giving you 2 columns (Distance to zone border [ARENA]) TOTAL and MEAN... click on one of those and go to PARAMETER PROPERTIES.... click on "mean" and hit the left arrow (getting rid of it - we only need "total" (aka cumulative).. then click on the DISTANCE TO ZONE BORDER tab... i can't remember what the zones are called in your file, but you want to move "Arena" to the left, and move the "platform zone" to the right.... then OK and run the analysis... 5x / sec, the animal's distance (in cm) to the edge of the platform is measured and all of these measurements are added up, producing the "cumulative distance to platform" parameter. | |||
<br> | |||
====Openfield Activity==== | |||
=====From Media Cruz to Ethovision:===== | |||
*Open Ethovision → new workspace: give it name *(can have multiple experiments in new workspace) → save → add an experiment → new experiment → name it → | |||
=====Activity Ethovision:===== | |||
*Design experiment: | |||
*Go to experiment → go to design → add what you desire | |||
*To Set up arena → digital video file → click box on "rectangle" icon on toolbar → draw box around bottom of field parameters (bins: lft to right , top to bottom = 1, 2, 3, 4 . Draw box. Drag arena so arrow in box → add arena → you'll get arena 2. Hit copy for arena 1 and paste box to bin 2. Repeat process for bins 3 & 4. | |||
*This set up will get you parameters for basic measures: distance moved & % time moved | |||
*To set up for: | |||
**(a) Rearings: set up computer to track animal, (computer tracks whether or not animal parameter gets half its size). Not 100% accurate. So as the computer scores, watch video for a few samples to make sure the computer is measuring the valid factor. | |||
**(b) how much time they spent in the corner (anxiety): set up zones: | |||
Zone → add zone definition → type "corner" → go to bins & make a rectangular parameter in corner of bins. Repeat for each corner of bin. Go to next arena (pull down tab on top right of screen) → add zone → repeat previous procedures for rest of arenas. | |||
*Once done setting up for data extraction: | |||
*Calibrate arena → click Add → draw line from one end of arena to other (49.5 cm) → ok. | |||
*To acquire data: → Hit play (bottom right of screen)→ go to tracking → go to processing → use gray scaling (if possible). (The subtraction: takes pic of bottom & looks at if pic changes). Click next tab (areas to search): use scan window. Go to last tab (click erosion & dilation). Go to Update detection variables: set low limit first: get the camera tracking just animal body and nothing more. (If you can't get it tracking all animals perfectly; might have to track them separately. | |||
*Go to tracking: Processing: area to search (size 25 x 25). Box around animal should be tighter. Keep adjusting tracking until it seems adequate. | |||
*Once tracking is set: hit "stop" → click on play as fast as possible and double-check tracking. | |||
*Go to Design: Yes → go to definitions → variable → show system variables → add Status, trail duration, arena, track file, → click ok → go to variables → user defined variables → id, (gender, whatever else you want, etc.) → go to values → add tracks → however many tracks you need (4x video numbers). | |||
*Acquire data: click "as fast as possible." Verify that video is starting at beginning. And go to tracking → trial protocol → set to 30 minutes instead of 5 min → Hit play up on tool bar → type in ID (use word "bunk" for bins that aren't tracking well if you plan on tracking them separately, and you can rerecord the other bins later after resetting the tracking for the bunk bins). | |||
*Visualize data: make sure the data's good. Can go to play & adjust the options (go fastest & autorepeat and show all points). Can get a whole screen shot. | |||
*To Analyze data: | |||
*To break data down into discrete time points: Data → Nesting → time window → keep "start of time" and "to end of time" selected → Go to interval → set for 3 min → ok → Right click and go to edit lay out → independent variables → add track, arena, etc. whatever you added → ok | |||
*Go to Analysis: Parameters → click what you desire to analyze: | |||
**Some commonly used: | |||
***(a) "in zone": right click on heading of selected parameter → parameter properties → move latency of .. to left → go to in zone & choose zones. | |||
***(b) for % time spent moving: choose the "Movement" parameter and have it output both "Total duration (%)" and "Total duration (s)". The "Total duration (%)" number will represent the total % of the 30 min trial (or 3 min bin) that the animal spent "moving" (as defined by the numbers in the "movement" tab). | |||
***c) for average walking speed: use the "Total duration (s)". | |||
****NOTE: the default "Velocity" parameter simply divides the total distance moved by the total length of the trial. This produces a number skewed toward the low end because it takes into account all of the time that the animal is sitting still. Therefore to determine average walking speed, divide total distance moved by "Total duration (s)" to determine the walking speed (in cm/s) *only* while the animal is actually walking... | |||
***c) for Distance moved: parameter properties: choose total distance moved | |||
*Once all parameters and their properties have been selected, click exclamation point on tool bar to extract data. | |||
== Water maze == | == Water maze == | ||
[[Image:BNL watermaze.jpg|left|thumb|px180|water maze tank and spatial cues]] | [[Image:BNL watermaze.jpg|left|thumb|px180|water maze tank and spatial cues]] | ||
| Line 127: | Line 182: | ||
===Time estimates=== | ===Time estimates=== | ||
* | '''Note''': All estimates assume tracking is properly set up BEFORE testing begins. | ||
Formulas take the total time needed for testing and simply divide by the number of animals present. | |||
All estimates assume MINIMAL complications, and are intended as gross estimates ONLY. | |||
Specific independent variables, like the nature of the brain damage presented by your animals CAN and WILL vary the amount of time needed. | |||
*Cued: 7-8min per animal | |||
*Spatial 1: 8-9min per animal | |||
*Probe 1/Spatial 2: 9-10+ min per animal | |||
*Probe 2/Spatial 3: 8.5-9.5 min per animal | |||
*Probe 3: 1-2 min per animal | |||
<br> | <br> | ||
==Rotarod== | |||
*Instructions for use | |||
**Turn the machine on from the back, on top of the electricity plug | |||
**Press cancel | |||
**Move arrow to experiment setup | |||
**Hit enter | |||
**Manipulate acceleration (accel.), start speed (s-sp) and acceleration interval (acc-in) to match protocol | |||
**Hit enter | |||
**Hit cancel | |||
**Go to run experiment | |||
**Hit enter | |||
**Press each channel key that you will use (ie CH1, CH2, etc..) to activate the lanes used | |||
**Place animals in the lane | |||
**When the animal falls, the top number is latency, and the bottom number speed (basically, ignore bottom number, only look at it while animal is running) | |||
**After the completion of the trial, hit cancel | |||
**Move arrow to run experiment | |||
**Repeat | |||
==Activity== | |||
*Instructions for setup and use | |||
**Make sure camera cords are plugged in (plug camera into yellow RCA input and electrical cord into electrical outlet) | |||
**Turn off all overhead lights (push the left on/off button in the light panel outside the door) | |||
**Place stage lights at all 4 corners of activity area | |||
**Double click media cruise on desktop | |||
**Make sure the boxes are within the recorded arena | |||
**Make sure box number corresponds to screen | |||
***Make sure that recording time is set to 30 minutes (click parameter button, check enable capture duration- enter 30 minutes) | |||
***Click format- make sure M-PEG1 is clicked | |||
***Click source- make sure the video input is “composite” | |||
***To control where the files are saved (or if there is no room left on the hard drive), click the folder icon | |||
**Place animals into appropriate boxes | |||
**Hit red circular record button | |||
**Recording will stop after time is finished | |||
**Save file in format: activity_day#_file#_a#_a#_a#_a# | |||
== Zero maze == | == Zero maze == | ||
See the Cincinatti Children Hospital's zero maze site [http://www.cincinnatichildrens.org/research/cores/abc/elevated.htm here] | See the Cincinatti Children Hospital's zero maze site [http://www.cincinnatichildrens.org/research/cores/abc/elevated.htm here] | ||
| Line 139: | Line 241: | ||
<br> | <br> | ||
== Forced swim test of depression == | == Forced swim test of depression == | ||
[http://www.natureprotocols.com/2007/12/13/animal_models_for_depressionli.php protocol] | [http://www.natureprotocols.com/2007/12/13/animal_models_for_depressionli.php protocol] | ||
Latest revision as of 11:19, 18 August 2009
Ethovision
Morris Water Maze Setup Instructions
- Go to Start → Ethovision
- File → new workspace → Name file → click Save
- File → new → experiment → name → Save
- Experiment → design → bottom tab (definitions)
- Variables → show system variable → click Status
- Variables → user defined variables → insert “ID” for “new label”
- Variables → user defined variables → insert “release point”
- Variable → user defined variables → insert “block”
- Variable → user defined variables → insert “platform”
- Variable → user defined variables → insert “trial type”
- Variable → show system variables → click “trial duration”
- Click bottom tab (values)
- Add tracks: click “track” → add tracks
- {non probe condition: 10 x n = # of tracks}
- {Probe Condition: n= # of tracks}
- Manually fill in: ID, release point, block, platform and trial type
- Experiment → arena definition → video source:
- Piccolo→ input channel → composite (BNC)
- TV standard → NTSC
- Acquired resolution → medium → ok
- Arena → add arena → name it → ok
- Click on circle button → click on two locations where the water meets the tank → pull the circle to fit correct location → pull the “talk box” arena X into circle
- Zone → zone definition → name it tank
- Click line tool button → divide it into four quadrants → click new tool button → name based on location (NE, NW, etc…) → drag the end of the talk box into tank
- Click paint bucket button to fill quadrant with color
- Make a fifth zone for platform location
- Modify based on platform location each day
- Bottom tab → calibration → add → click ok
- Drag the line from one side of the tank to the other → enter 110 cm → ok → ok
- Experiment → acquire data → tracking → trial protocol → recording duration: enter 1:00 → click ok
- Tracking → processing → detection method:
- For rat: object intensity is brighter
- For mouse: object intensity is darker
- Image filtering → click erosion and dilation → click ok
- Click F4 → modify as necessary
- Click arrow “play” button to record
- Click square “stop” button to stop after recording is finished
Ethovision Data Extraction Instructions
Cumulative Distance to Platform
- go to ADD PARAMETER > DISTANCE TO ZONE BORDER... you'll notice that it defaults to giving you 2 columns (Distance to zone border [ARENA]) TOTAL and MEAN... click on one of those and go to PARAMETER PROPERTIES.... click on "mean" and hit the left arrow (getting rid of it - we only need "total" (aka cumulative).. then click on the DISTANCE TO ZONE BORDER tab... i can't remember what the zones are called in your file, but you want to move "Arena" to the left, and move the "platform zone" to the right.... then OK and run the analysis... 5x / sec, the animal's distance (in cm) to the edge of the platform is measured and all of these measurements are added up, producing the "cumulative distance to platform" parameter.
Openfield Activity
From Media Cruz to Ethovision:
- Open Ethovision → new workspace: give it name *(can have multiple experiments in new workspace) → save → add an experiment → new experiment → name it →
Activity Ethovision:
- Design experiment:
- Go to experiment → go to design → add what you desire
- To Set up arena → digital video file → click box on "rectangle" icon on toolbar → draw box around bottom of field parameters (bins: lft to right , top to bottom = 1, 2, 3, 4 . Draw box. Drag arena so arrow in box → add arena → you'll get arena 2. Hit copy for arena 1 and paste box to bin 2. Repeat process for bins 3 & 4.
- This set up will get you parameters for basic measures: distance moved & % time moved
- To set up for:
- (a) Rearings: set up computer to track animal, (computer tracks whether or not animal parameter gets half its size). Not 100% accurate. So as the computer scores, watch video for a few samples to make sure the computer is measuring the valid factor.
- (b) how much time they spent in the corner (anxiety): set up zones:
Zone → add zone definition → type "corner" → go to bins & make a rectangular parameter in corner of bins. Repeat for each corner of bin. Go to next arena (pull down tab on top right of screen) → add zone → repeat previous procedures for rest of arenas.
- Once done setting up for data extraction:
- Calibrate arena → click Add → draw line from one end of arena to other (49.5 cm) → ok.
- To acquire data: → Hit play (bottom right of screen)→ go to tracking → go to processing → use gray scaling (if possible). (The subtraction: takes pic of bottom & looks at if pic changes). Click next tab (areas to search): use scan window. Go to last tab (click erosion & dilation). Go to Update detection variables: set low limit first: get the camera tracking just animal body and nothing more. (If you can't get it tracking all animals perfectly; might have to track them separately.
- Go to tracking: Processing: area to search (size 25 x 25). Box around animal should be tighter. Keep adjusting tracking until it seems adequate.
- Once tracking is set: hit "stop" → click on play as fast as possible and double-check tracking.
- Go to Design: Yes → go to definitions → variable → show system variables → add Status, trail duration, arena, track file, → click ok → go to variables → user defined variables → id, (gender, whatever else you want, etc.) → go to values → add tracks → however many tracks you need (4x video numbers).
- Acquire data: click "as fast as possible." Verify that video is starting at beginning. And go to tracking → trial protocol → set to 30 minutes instead of 5 min → Hit play up on tool bar → type in ID (use word "bunk" for bins that aren't tracking well if you plan on tracking them separately, and you can rerecord the other bins later after resetting the tracking for the bunk bins).
- Visualize data: make sure the data's good. Can go to play & adjust the options (go fastest & autorepeat and show all points). Can get a whole screen shot.
- To Analyze data:
- To break data down into discrete time points: Data → Nesting → time window → keep "start of time" and "to end of time" selected → Go to interval → set for 3 min → ok → Right click and go to edit lay out → independent variables → add track, arena, etc. whatever you added → ok
- Go to Analysis: Parameters → click what you desire to analyze:
- Some commonly used:
- (a) "in zone": right click on heading of selected parameter → parameter properties → move latency of .. to left → go to in zone & choose zones.
- (b) for % time spent moving: choose the "Movement" parameter and have it output both "Total duration (%)" and "Total duration (s)". The "Total duration (%)" number will represent the total % of the 30 min trial (or 3 min bin) that the animal spent "moving" (as defined by the numbers in the "movement" tab).
- c) for average walking speed: use the "Total duration (s)".
- NOTE: the default "Velocity" parameter simply divides the total distance moved by the total length of the trial. This produces a number skewed toward the low end because it takes into account all of the time that the animal is sitting still. Therefore to determine average walking speed, divide total distance moved by "Total duration (s)" to determine the walking speed (in cm/s) *only* while the animal is actually walking...
- c) for Distance moved: parameter properties: choose total distance moved
- Some commonly used:
- Once all parameters and their properties have been selected, click exclamation point on tool bar to extract data.
Water maze




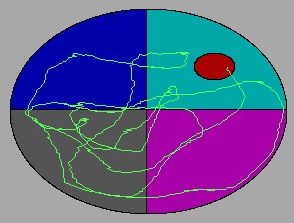
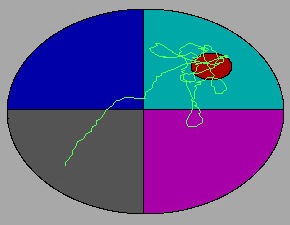



Apparatus
The water maze consists of a metal pool (110 cm diameter) in a well-lit room filled to within 15 cm (???) of the upper edge with water made opaque by the addition of white non-toxic tempera paint. The pool contains a round "escape" platform (11 cm diameter) that the animal can step on to get out of the water. Water temperature is recorded at the beginning of each day of testing and adjusted to within 65-75 degrees F as necessary. After each week of testing, the water maze tank is completely drained and sanitized with Quadracide (2 oz / gal H2O).
General methods
For each trial, an animal is released nose against the wall into the pool at one of four release points and allowed to find the platform. The animals are given 10 trials per day in 5 blocks of 2 consecutive trials with a 10 min inter-block interval. All trials last a maximum of 60 s, at which point the animal is manually guided to the platform. An overhead camera records the animals’ swim paths, allowing for quantification of distance, latency, proximity to target, and swimming speed by a computerized tracking system (Ethovision 3.1). As performance improves, escape latency and swim path length generally decrease.
Cued task
For the cued version of this task, the surface of the escape platform is visible (5 mm above the surface of the water), and a 20 cm tall pole is placed on top of the platform to make its location even more obvious. The location of the platform changes for each block of trials. The animals are released into the pool opposite the location of the platform for that trial, and are allowed to remain on the platform for 5 s. Animals that display behaviors inappropriate for water maze testing (including spinning, thygmotaxic navigation around the perimeter of the pool, and inability to swim) are removed from the water maze study. The maximum amount of time that an animal will swim over the course of one day is 10 min (1 min x 10 trials).
Spatial task
This task is identical to the cued water maze task, except that the surface of the escape platform is submerged 1 cm below the surface of the water and the marker removed, so the animal must find the platform based on its relationship to the spatial cues rather than direct visualization. It will remain in the same location for all 10 trials, and animals are release from all 4 release points at least twice.
Probe trials
After the final spatial trial, the platform is removed and the animal is allowed to swim for 60 s.
Sample counter-balanced design
- 1st 2 SPATIAL trials of each day should be from "far" release points for proper analysis of working memory
- for trials 3-10, counter-balance close and far for each block of 2 trials, with all 4 release points being used for each block of 4 trials
- Day 1 - Cued
- Trials 1a and 1b: platform NE quad, release SW
- Trials 2a and 2b: platform SW quad, release NE
- Trials 3a and 3b: platform NW quad, release SE
- Trials 4a and 4b: platform SE quad, release NW
- Trials 5a and 5b: platform NE quad, release SW
- Day 2 - Spatial location 1 (NE - center of quadrant)
- Trial 1a: S (far)
- Trial 1b: W (far)
- Trial 2a: N (close)
- Trial 2b: S (far)
- Trial 3a: W (far)
- Trial 3b: E (close)
- Trial 4a: N (close)
- Trial 4b: W (far)
- Trial 5a: S (far)
- Trial 5b: E (close)
- Day 3 - Probe 1 and spatial location 2 (W - closer to edge)
- Probe trial: no platform, release SW
- Trial 1a: NE (far)
- Trial 1b: SE (far)
- Trial 2a: NW (close)
- Trial 2b: NE (far)
- Trial 3a: SE (far)
- Trial 3b: SW (close)
- Trial 4a: SW (close)
- Trial 4b: NE (far)
- Trial 5a: SE (far)
- Trial 5b: NW (close)
- Day 4 - Probe 2 and spatial location 3 (E - closer to center)
- Probe trial: no platform, release E
- Trial 1a: NW (far)
- Trial 1b: SW (far)
- Trial 2a: SW (far)
- Trial 2b: NE (close)
- Trial 3a: SE (close)
- Trial 3b: NW (far)
- Trial 4a: SW (far)
- Trial 4b: SE (close)
- Trial 5a: NE (close)
- Trial 5b: NW (far)
- Day 5 - Probe 3: no platform, release E
Notes
- Note that the behavioral tests described do not require the filming or photographing of the animals. The only information that is collected by the camera/computer system is the current X-Y coordinate location of the animal within the visual field. Therefore, no procedures will need to be implemented to secure photographic tapes, films, or files.
Tips, tricks, etc
- having 2 people run the test is preferred
- have lights properly set up before running experiment
Movies
- ?
Time estimates
Note: All estimates assume tracking is properly set up BEFORE testing begins. Formulas take the total time needed for testing and simply divide by the number of animals present. All estimates assume MINIMAL complications, and are intended as gross estimates ONLY. Specific independent variables, like the nature of the brain damage presented by your animals CAN and WILL vary the amount of time needed.
- Cued: 7-8min per animal
- Spatial 1: 8-9min per animal
- Probe 1/Spatial 2: 9-10+ min per animal
- Probe 2/Spatial 3: 8.5-9.5 min per animal
- Probe 3: 1-2 min per animal
Rotarod
- Instructions for use
- Turn the machine on from the back, on top of the electricity plug
- Press cancel
- Move arrow to experiment setup
- Hit enter
- Manipulate acceleration (accel.), start speed (s-sp) and acceleration interval (acc-in) to match protocol
- Hit enter
- Hit cancel
- Go to run experiment
- Hit enter
- Press each channel key that you will use (ie CH1, CH2, etc..) to activate the lanes used
- Place animals in the lane
- When the animal falls, the top number is latency, and the bottom number speed (basically, ignore bottom number, only look at it while animal is running)
- After the completion of the trial, hit cancel
- Move arrow to run experiment
- Repeat
Activity
- Instructions for setup and use
- Make sure camera cords are plugged in (plug camera into yellow RCA input and electrical cord into electrical outlet)
- Turn off all overhead lights (push the left on/off button in the light panel outside the door)
- Place stage lights at all 4 corners of activity area
- Double click media cruise on desktop
- Make sure the boxes are within the recorded arena
- Make sure box number corresponds to screen
- Make sure that recording time is set to 30 minutes (click parameter button, check enable capture duration- enter 30 minutes)
- Click format- make sure M-PEG1 is clicked
- Click source- make sure the video input is “composite”
- To control where the files are saved (or if there is no room left on the hard drive), click the folder icon
- Place animals into appropriate boxes
- Hit red circular record button
- Recording will stop after time is finished
- Save file in format: activity_day#_file#_a#_a#_a#_a#
Zero maze
See the Cincinatti Children Hospital's zero maze site here
Apparatus
The zero maze consists of a plastic ring, 100 cm outer diameter, 10 cm wide, with 35 cm walls enclosing 2 opposing quadrants.
General methods
