Hartman Behavioral Neuroscience Lab:Protocols
Ethovision
water maze
- Go to Start → Ethovision
- File → new workspace → Name file → click Save
- File → new → experiment → name → Save
- Experiment → design → bottom tab (definitions)
- Variables → show system variable → click Status
- Variables → user defined variables → insert “ID” for “new label”
- Variables → user defined variables → insert “release point”
- Variable → user defined variables → insert “block”
- Variable → user defined variables → insert “platform”
- Variable → user defined variables → insert “trial type”
- Variable → show system variables → click “trial duration”
- Click bottom tab (values)
- Add tracks: click “track” → add tracks
- {non probe condition: 10 x n = # of tracks}
- {Probe Condition: n= # of tracks}
- Manually fill in: ID, release point, block, platform and trial type
- Experiment → arena definition → video source:
- Piccolo→ input channel → composite (BNC)
- TV standard → NTSC
- Acquired resolution → medium → ok
- Arena → add arena → name it → ok
- Click on circle button → click on two locations where the water meets the tank → pull the circle to fit correct location → pull the “talk box” arena X into circle
- Zone → zone definition → name it tank
- Click line tool button → divide it into four quadrants → click new tool button → name based on location (NE, NW, etc…) → drag the end of the talk box into tank
- Click paint bucket button to fill quadrant with color
- Make a fifth zone for platform location
- Modify based on platform location each day
- Bottom tab → calibration → add → click ok
- Drag the line from one side of the tank to the other → enter 110 cm → ok → ok
- Experiment → acquire data → tracking → trial protocol → recording duration: enter 1:00 → click ok
- Tracking → processing → detection method:
- For rat: object intensity is brighter
- For mouse: object intensity is darker
- Image filtering → click erosion and dilation → click ok
- Click F4 → modify as necessary
- Click arrow “play” button to record
- Click square “stop” button to stop after recording is finished
Water maze




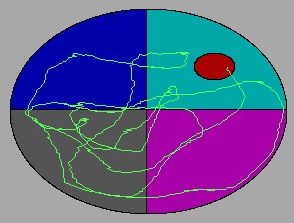
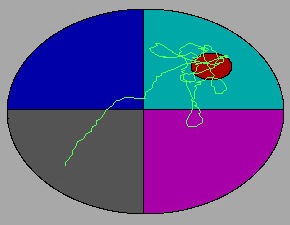



Apparatus
The water maze consists of a metal pool (110 cm diameter) in a well-lit room filled to within 15 cm (???) of the upper edge with water made opaque by the addition of white non-toxic tempera paint. The pool contains a round "escape" platform (11 cm diameter) that the animal can step on to get out of the water. Water temperature is recorded at the beginning of each day of testing and adjusted to within 65-75 degrees F as necessary. After each week of testing, the water maze tank is completely drained and sanitized with Quadracide (2 oz / gal H2O).
General methods
For each trial, an animal is released nose against the wall into the pool at one of four release points and allowed to find the platform. The animals are given 10 trials per day in 5 blocks of 2 consecutive trials with a 10 min inter-block interval. All trials last a maximum of 60 s, at which point the animal is manually guided to the platform. An overhead camera records the animals’ swim paths, allowing for quantification of distance, latency, proximity to target, and swimming speed by a computerized tracking system (Ethovision 3.1). As performance improves, escape latency and swim path length generally decrease.
Cued task
For the cued version of this task, the surface of the escape platform is visible (5 mm above the surface of the water), and a 20 cm tall pole is placed on top of the platform to make its location even more obvious. The location of the platform changes for each block of trials. The animals are released into the pool opposite the location of the platform for that trial, and are allowed to remain on the platform for 5 s. Animals that display behaviors inappropriate for water maze testing (including spinning, thygmotaxic navigation around the perimeter of the pool, and inability to swim) are removed from the water maze study. The maximum amount of time that an animal will swim over the course of one day is 10 min (1 min x 10 trials).
Spatial task
This task is identical to the cued water maze task, except that the surface of the escape platform is submerged 1 cm below the surface of the water and the marker removed, so the animal must find the platform based on its relationship to the spatial cues rather than direct visualization. It will remain in the same location for all 10 trials, and animals are release from all 4 release points at least twice.
Probe trials
After the final spatial trial, the platform is removed and the animal is allowed to swim for 60 s.
Sample counter-balanced design
- 1st 2 SPATIAL trials of each day should be from "far" release points for proper analysis of working memory
- for trials 3-10, counter-balance close and far for each block of 2 trials, with all 4 release points being used for each block of 4 trials
- Day 1 - Cued
- Trials 1a and 1b: platform NE quad, release SW
- Trials 2a and 2b: platform SW quad, release NE
- Trials 3a and 3b: platform NW quad, release SE
- Trials 4a and 4b: platform SE quad, release NW
- Trials 5a and 5b: platform NE quad, release SW
- Day 2 - Spatial location 1 (NE - center of quadrant)
- Trial 1a: S (far)
- Trial 1b: W (far)
- Trial 2a: N (close)
- Trial 2b: S (far)
- Trial 3a: W (far)
- Trial 3b: E (close)
- Trial 4a: N (close)
- Trial 4b: W (far)
- Trial 5a: S (far)
- Trial 5b: E (close)
- Day 3 - Probe 1 and spatial location 2 (W - closer to edge)
- Probe trial: no platform, release SW
- Trial 1a: NE (far)
- Trial 1b: SE (far)
- Trial 2a: NW (close)
- Trial 2b: NE (far)
- Trial 3a: SE (far)
- Trial 3b: SW (close)
- Trial 4a: SW (close)
- Trial 4b: NE (far)
- Trial 5a: SE (far)
- Trial 5b: NW (close)
- Day 4 - Probe 2 and spatial location 3 (E - closer to center)
- Probe trial: no platform, release E
- Trial 1a: NW (far)
- Trial 1b: SW (far)
- Trial 2a: SW (far)
- Trial 2b: NE (close)
- Trial 3a: SE (close)
- Trial 3b: NW (far)
- Trial 4a: SW (far)
- Trial 4b: SE (close)
- Trial 5a: NE (close)
- Trial 5b: NW (far)
- Day 5 - Probe 3: no platform, release E
Notes
- Note that the behavioral tests described do not require the filming or photographing of the animals. The only information that is collected by the camera/computer system is the current X-Y coordinate location of the animal within the visual field. Therefore, no procedures will need to be implemented to secure photographic tapes, films, or files.
Tips, tricks, etc
- having 2 people run the test is preferred
- have lights properly set up before running experiment
Movies
- ?
Time estimates
- ?
Zero maze
See the Cincinatti Children Hospital's zero maze site here
Apparatus
The zero maze consists of a plastic ring, 100 cm outer diameter, 10 cm wide, with 35 cm walls enclosing 2 opposing quadrants.
General methods
Forced swim test of depression
Plasma collection (survival)
- with some practice and a little isoflurane, it's possible to collect ~500µl of blood via eye-bleed in under 1 minute:
- have prepped:
- 2 labeled bullet vials per animal (on ice)
- 1 heparinized capillary tube per mouse
- insert tube behind the eye (into the retro-orbital socket) and collect into vial
- centrifuge for 5m @ 14,000 rpm
- pipette plasma from top of vial into fresh vial
- store in -80 freezer
- have prepped:
Plasma collection (terminal)
- blood is ~50% plasma (the liquid portion that has not been clotted, lysed, etc - vs. serum)
- can get ~700µl / mouse
- have prepped:
- 2 labeled bullet vials per animal (on ice)
- 1cc syringe w/ 23g needle
- 1 per animal
- plunge each 3x into .5 molar EDTA
- just before perfusion, take blood from heart @ same poke-point (needle's bevel UP)
- remove needle before evacuating blood into vial (to avoid rupturing cells)
- centrifuge for 5m @ <14,000 rpm
- pipette plasma from top of vial into fresh vial
- store in -80 freezer
Simple perfusion
- Obtain:
- 2 buckets of ice
- 50 mg/ml nembutal (Na pentobarb)
- 9x13 pyrex dish w/ styrofoam block & syringe tips to hold limbs
- body bag
- pump (set @ 35) - 5-7mls/min
- 1L nalgene jar of 1x PBS in ice bucket
- 100ml 10x
- 900ml millipore water
- 3-4 ml heparin (to thin blood)
- place front tube of pump into PBS (tape & get bubbles out)
- small jars of 4% paraformaldehyde on ice for brain storage
- For 100ml 4% (150ml good for 10 mice) - make in .3L graduated cylinder:
- 50ml phosphate (PO4) buffer (.25 molar)
- 30ml double distilled h2o
- 20ml 20% paraformaldehyde
- For 100ml 4% (150ml good for 10 mice) - make in .3L graduated cylinder:
- tools in top L drawer (rinse & put away when done):
- 1 hemos for flap hold
- scissors for body cut
- scissors for skull / heart cut
- tweezers to hold heart
- Procedure:
- knock out animal w/ ip injection (.5cc)
- perfuse
- dissect brain out
- soak in 4% for ~24 hrs
- soak in 30% sucrose til it sinks
- cut & stain
Histology
Cresyl Violet staining (old school)
- Fix:
- Place dry slides into a carrying tray and put into a dish with approximately 1/8" of formalin in the bottom.
- Note: slides should not come into direct contact with formalin
- Cover the tray and place in the oven (50-60Ε C) for 20-60 minutes
- Place dry slides into a carrying tray and put into a dish with approximately 1/8" of formalin in the bottom.
- Stain:
- Place the carrying tray into the following solutions for the specified minimum times:
- Note: tap off excess solution before the next step
- distilled water 5 min.
- 70% alcohol 5 min.
- 95% alcohol 5 min.
- 100% alcohol 10 min.
- xylenes 15 min.
- 100% alcohol 10 min.
- 95% alcohol 5 min.
- 70% alcohol 5 min.
- distilled water 5 min.
- Note: the above steps remove fat and water, then replace the water
- stain 2-4 min.
- distilled water rinse
- 70% alcohol 30-60 sec.
- 95% alcohol until differentiated (hours to overnight)
- 100% alcohol rinse
- xylenes at least 10 min. (until cover slipped)
- Cover Slipping:
- Do not remove slides from xylenes until ready to cover slip
- Note: DO NOT LET THE SLIDE DRY OUT
- Place approximately 1 drop of synthetic resin between each section on the slide
- Smooth out the resin with an applicator stick with the last stroke being away from the labeled end. This will leave a mound of resin at one end of the slide.
- Put the cover slip on, beginning with the end of the slip over the resin mound
- gently bend the slip and lay it down progressively from the section end of the slide to the label end of the slide
- Gently push any air bubbles to the side, and out of the cover slip
- Place the finished slides onto a drying board and let dry completely (may takes weeks to months)
- Scrape with razor blade / clean with xylenes
- Do not remove slides from xylenes until ready to cover slip
- General Notes:
- Sections (in ice cube trays - before mounting) may be left in water for 24 hours, but only in an emergency!
- The process may be reversed at any point (including after cover slipping) if needed
- Slides may stay in xylenes for up to 48 hours (make sure slides are completely covered!)
- Slides may be viewed under a microscope after a minimum of one day, and then only under the projector microscope until completely dry
- Alcohol Solutions:
- Do NOT use 100% alcohol to mix lower percentages, it is comparatively very expensive
- 70% from 95%:
- pour 800cc of 95% alcohol into a large jar
- add enough distilled water to make 1085.7cc of solution
- 80% from 95%:
- pour 800cc of 95% alcohol into a large jar
- add enough distilled water to make 950cc of solution
- 70% from 80%:
- pour 700cc of 80% alcohol into a large jar
- add enough distilled water to make 800cc of solution
Recipes
Cresyl Violet stain
- make a 0.2% solution of Cresyl violet acetate and distilled water
- 2g cresyl violet acetate - add enough distilled water to bring to 1000ml
- shake well and filter (use vacuum filter)
Saline
- 8.7 g sodium chloride : 1 liter distilled water
Formalin
- 9 parts saline : 1 part formaldehyde
Sucrose Formalin
- dissolve 900 g sugar in 2175 ml distilled water
- add 300 ml concentrated formalin (e.g., 10% formalin)
- refrigerate
Gelatinizing Slides
- dissolve 6.6 g powdered gelatin in 200 cc distilled water @ 45ΕC
- while stirring, add 200 cc 80% alcohol (168.42 cc 95%)
- submerge slides for 2 minutes in a wheaton jar that contains gelatin solution
- place slide tray in 50-60ΕC oven for 30-60 minutes, or until dry
Injections
Nembutal
- 35 mg / kg of rat
- Example @ 50 mg / cc:
- 395 g rat = .395 kg
- .395 x 35 = 13.8 mg for this rat
- 13.8 / 50 = .276 cc of Nembutal
- Example @ 50 mg / cc:
Sodium Pentobarbitol
- Anaesthetic:
- I.V. 25 mg / kg
- I.P. 50 mg / kg (25-30) ???
- fairly long duration
- tachycardia, depression of cardiovascular and spinal cord reflexes
