Haynes:NewProtocol: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
Rene M Davis (talk | contribs) No edit summary |
Rene M Davis (talk | contribs) |
||
| Line 22: | Line 22: | ||
[[Image:Slide_3_pdf.png|500px|Gibson Assembly primers]]<br> | [[Image:Slide_3_pdf.png|500px|Gibson Assembly primers]]<br> | ||
PCR mix<br> | |||
15ul of Gibson Assembly Mix | |||
(I will add the recipe soon, but for now, find 15ul aliquotes in PCR tubes in a clearly labeled bag in the -20C freezer door) | |||
==Procedure== | ==Procedure== | ||
Revision as of 11:30, 26 October 2012
Gibson Assembly
Overview
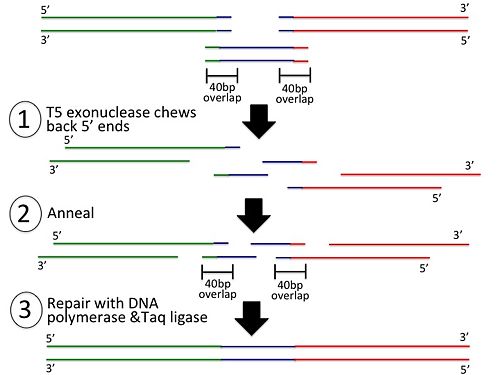
The Gibson Assembly method allows you to assemble multiple DNA parts in just 2 steps.
In the first step, 20 basepair overlaps are added to the sequences to be assembled:
In the second step, the PCR products are added to the Gibson Assembly Mix:
Materials
Designing primers:
PCR mix
15ul of Gibson Assembly Mix
(I will add the recipe soon, but for now, find 15ul aliquotes in PCR tubes in a clearly labeled bag in the -20C freezer door)
Procedure
- In a PCR tube, mix the components on ice in the order they are listed above.
- Perform thermocycling program
- 95 °C 5 min
- 95 °C 30 s
- TH 30 s
- 72 °C 1 min for each 1 kb PCR product
- Repeat steps 2-4 a total of 12-36 times (24 is standard).
- 72 °C 5 min
- 12 °C hold
Notes
Please feel free to post comments, questions, or improvements to this protocol. Happy to have your input!
- List troubleshooting tips here.
- You can also link to FAQs/tips provided by other sources such as the manufacturer or other websites.
- Anecdotal observations that might be of use to others can also be posted here.
Please sign your name to your note by adding '''*~~~~''': to the beginning of your tip.
References
Relevant papers and books
- Gibson, DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO (2009) - Nature Methods 6(5) 343-5 PMID 19363495
Contact
- René at rene.davis at asu dot edu
or instead, discuss this protocol.