IGEM:Caltech/2007/Project/Q: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
No edit summary |
|||
| Line 18: | Line 18: | ||
Once Q has modified the RNA polymerase, the polymerase begins to transcribe genes beyond tR'. These genes are crucial to the production of new phage components, such as tails and heads (protein capsules). Once these genes are expressed, the phage is committed to the lytic pathway. | Once Q has modified the RNA polymerase, the polymerase begins to transcribe genes beyond tR'. These genes are crucial to the production of new phage components, such as tails and heads (protein capsules). Once these genes are expressed, the phage is committed to the lytic pathway. | ||
[[Image:Bacteriophage_lambda_genome.png|thumb|Schematic representation of the genome of the bacteriophage lambda. Q alters RNA polymerase and allows transcription of genes coding for heads and tails production.]] | |||
[[Image:Q_changes.jpg|center|changes brought on by the Q protein]] | [[Image:Q_changes.jpg|center|changes brought on by the Q protein]] | ||
| Line 26: | Line 28: | ||
==Status and Future Plans== | ==Status and Future Plans== | ||
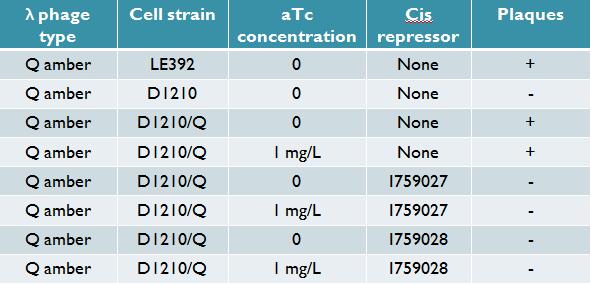
The Q constructs, consisting of promoter pTet, spacer, Q gene, double terminator, constitutive promoter, tetR gene, and double terminator have been made and sequence verified. There are two version of the construct, one with a medium-strength constitutive promoter driving tetR, and one with a strong constitutive promoter. Titration trials with Q amber mutants have shown that expression of Q under tetR repression using the medium-strength promoter is leaky but inducible with anhydrotetracycline (aTc). Replacing the spacer with various cis-repressor sequences has shut down the leaky repression. | The Q constructs, consisting of promoter pTet, spacer, Q gene, double terminator, constitutive promoter, tetR gene, and double terminator have been made and sequence verified. There are two version of the construct, one with a medium-strength constitutive promoter driving tetR, and one with a strong constitutive promoter. Titration trials with Q amber mutants have shown that expression of Q under tetR repression using the medium-strength promoter is leaky but inducible with anhydrotetracycline (aTc). Replacing the spacer with various cis-repressor sequences has shut down the leaky repression. | ||
[[Image:Q_results.jpg|center|Titering results for Q construct without cis repressor]] | |||
[[Image:Q results2.jpg|center|Titering results for Q construct with 2 successful cis repressors]] | |||
Future work on Q includes titering the construct featuring the strong constitutive promoter, introducing the conjugate trans activators to Q construct variants containing cis repressors, and quantitatively measuring the effects of aTc concentration on cell lysis rates. | Future work on Q includes titering the construct featuring the strong constitutive promoter, introducing the conjugate trans activators to Q construct variants containing cis repressors, and quantitatively measuring the effects of aTc concentration on cell lysis rates. | ||
| Line 31: | Line 37: | ||
[[Image:Q_Construct.jpg|The Q construct used to determine concentration of Q necessary for lytic pathway induction.]] | [[Image:Q_Construct.jpg|The Q construct used to determine concentration of Q necessary for lytic pathway induction.]] | ||
|} | |} | ||
</div> | </div> | ||