IGEM:Harvard/2006/Adaptamers/Notebook: Difference between revisions
(→8/2) |
(→8/1) |
||
| Line 487: | Line 487: | ||
T35+S35 must be heavy enough to not run too far away while the aptamer region is coming off thrombin, unlike T0 (the pure thrombin aptamer). Running the gel for 1 hour may have made a difference, too. Maybe we'll see an even better shift using T50+S50. | T35+S35 must be heavy enough to not run too far away while the aptamer region is coming off thrombin, unlike T0 (the pure thrombin aptamer). Running the gel for 1 hour may have made a difference, too. Maybe we'll see an even better shift using T50+S50. | ||
=Week 9= | =Week 9= | ||
Revision as of 13:43, 16 August 2006
Notebook
<calendar> name=IGEM:Harvard/2006/Adaptamers/Notebook date=2006/07/01 view=threemonths format=%name/%year-%month-%day weekstart=0 </calendar>
Week 4
7/7
Adaptamers:
We've received the short DNA sequences that we'll be using. We are waiting for purified streptavidin protein to come in. The main question is what we will use in our gel shift assay. We cannot use 32P, so our alternatives are:
Invitrogen EMSA kit (cat. E33075) $224. enough for 10 minigel assays.
Roche DIG gel shift (cat. 03353591910) $453. enough for 20 labeling rxns, 200 gel shift rxns, 20 blots, and 20 control rxns. Don't know what 'gel shift reactions' are. Also need nylon membranes, DIG wash and buffer set.
LightShift Chemiluminescent EMSA Kit (prod. # 20148) $349. Enough for 100 binding reactions (??), detection reagants for 800cm^2 membrane.
The first kit uses fluorescent dyes. We would need to buy a $600 filter for the gel imager to use it. The second two involve blotting membranes. Since this project will probably involve trying out a few types of aptamer sequences and many types of intermediate sequences between the two aptamer ends, the latter two, while supposedly more reliable, will likely be too costly. Licor also has a system based on fluorescent dyes but it looks like you buy the parts separately...
Alain suggested that all adaptamers that we create have a sequence that can bind a complementary short sequence; we'd order a lot of labeled short complementary sequence. Sounds like a good idea, as long as those short sequences do not interfere with the aptamer binding.
Week 5
7/11
Shawn and William suggested that for an initial assay, it'd be easy to just stain protein with Coomassie blue. So that's what we attempted.
We ran two 10-20% native polyacrylamide gels at 15 V overnight; the first used some lanes from the Nano group's gel. Visualization to come tomorrow.
The composition of what we added to each line is below; materials were added and incubated for 30 minutes in 2 uL total volume for lanes 1-11, 1-12 prior to dilution to 10 uL including addition of loading dye. Lanes 2-1 to 2-9 were incubated for an hour; longer incubation carried out since DNA basepairing reactions for lanes 2-10 to 2-12 were carried out for 30 minutes using results from 30-minute incubations of lanes 2-1 to 2-9. This incubation time should not be significant.
Materials used: Thrombin: a 2 uM solution of Thrombin. Streptavidin: a 2 uM solution of Streptavidin. Thromb5, Thromb20, Thromb35, Thromb50, Strep5, Strep20, Strep35, Strep50 are described in Plan section above. Bock's buffer is a reaction buffer for DNA-protein binding, used in several papers (see Nano group's page). 10X dye is a few flakes of bromophenol blue + 500 uL Bock's + 500 uL glycerol.
All quantities in uL.
| 1-11 | 1-12 | 2-1 | 2-2 | 2-3 | 2-4 | 2-5 | 2-6 | 2-7 | 2-8 | 2-9 | 2-10 | 2-11 | 2-12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombin | .5 | .5 | .5 | 1 | 1 | 1 | ||||||||
| Streptavidin | .5 | .5 | 1 | 1 | 1 | |||||||||
| Thromb5 | 1 | 1 | ||||||||||||
| Thromb20 | 2 | |||||||||||||
| Thromb35 | 2 | |||||||||||||
| Thromb50 | 2 | |||||||||||||
| Strep5 | 2 | |||||||||||||
| Strep20 | 2 | |||||||||||||
| Strep35 | 2 | |||||||||||||
| Strep50 | 2 | |||||||||||||
| 4X Bock's Buffer (see Nanostructure page) | .5 | .5 | .5 | 1 | 1 | 1 | .5 | .5 | 1 | 1 | 1 | |||
| H2O | 1 | 1 | ||||||||||||
| 2-2 solution | 2 | |||||||||||||
| 2-3 solution | 2 | |||||||||||||
| 2-4 solution | 2 | |||||||||||||
| 2-7 | 2 | |||||||||||||
| 2-8 | 2 | |||||||||||||
| 2-9 | 2 | |||||||||||||
| Bock's 1X buffer | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 5 | 5 | 5 |
| 10X dye | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Ran 2 10-20% polyacrylamide gels along with nano group.
7/12
The gels came out completely blank. Bummer. Potential problems were protein concentration, pH of reaction buffer, and voltage. Different concentrations were tried out by the nanogroup to no success.
Update: The machines were never turned on. Double-bummer.
7/13
For now, we will
1) debug SDS-PAGE with the nanos. 2) Search for possible cell surface protein targets for which aptamers have been developed (and have published sequences).
We heard back from the group that had created Lipopolysaccharide aptamers (that cand bind the E. coli cell surface). The group only created a polyclonal batch and never got specific aptamers sequenced before funding was cut. Unfortunate.
Nanos have gotten protein to show up on a gel; they ran it for 15 minutes at 120 V.
7/14
7/12 experiment essentially repeated. Alain kindly imaged the gels; results are shown in Week 5 presentation slides.
Week 6
7/17
Attempted to stain gels from 7/14 with EtBr. Gels were completely blank. The nanostructure group has since confirmed that staining with EtBr following staining with Coomassie Blue does not work.
7/18
Ran a gel with multiple lanes of the same condition in order to have separate visualizations of DNA and protein.
Lanes are as follow:
2: BSA
3: thrombin in BSA
4: thrombin + T5
5: streptavidin
6: streptavidin + S5
7: streptavidin + biotinylated oligos
8: 1 kb ladder
9: 5S
10: 5S + strep
11: 5T
12: 5T + thromb
Where combinations are noted, 4 picomoles DNA + 2 picomoles protein were incubated for 30 minutes in a total reaction volume of 4 uL. Following incubations, solutions were diluted to 10uL including loading dye and run on a 12% polyacrylamide gel at 120 V for 1.5 hours.
Lanes 1-7 were stained with Coomassie Blue; lanes 8-12 with EtBr.
The lane with BSA clearly showed three bands, disagreeing with the results of the Nanostructure group, which suggested that only the bottom-most band belonged to BSA, and the others to thrombin. It was hoped that we had simply added thrombin to the BSA lane, but results from 7/21 showed that BSA in fact was responsible for all the bands.
No shift was observed for thrombin, although both DNA and aptamer bands appeared darker in lanes where they were incubated together, which is inconsistent with binding. Streptavidin only showed very lightly and not at all in the lane with biotin; perhaps streptavidin did bind biotin.
One curiosity is the bands stained with EtBr. S5 is more than double the length of T5, yet they appeared to migrate to the same location. I initially thought that the band somehow matched with the loading dye, but visual inspection showed that this was not the case.
7/21
Following continued negative results obtained by the nanostructure group, we repeated most of 7/18's gel at extremely high concentrations with more attention to the positive control of streptavidin and biotin binding. The rationale was that if binding did not occur at these concentrations, then there was something quite wrong with either our materials or procedure. Aiding our confidence in this experiment, more highly-purified samples of the longer aptamers arrived this week (in particular, S5). Incubations were done in total volumes of 9uL.
1: SeeBlue Plus 2 ladder
2: BSA
3: thrombin (100 pmol)
4: thrombin (100 pmol) + T5 (200 pmol)
5: streptavidin (94.5 pmol)
6: streptavidin (94.5 pmol) + S5 (200 pmol)
7: streptavidin (94.5 pmol) + biotinylated oligos (200 pmol)
8: 1kb dna ladder
9: biotinylated oligos (200 pmol)
10: biotinylated oligos (200 pmol) + streptavidin (94.5 pmol)
11: S5 (200 pmol)
12: S5 (200 pmol) + streptavidin (94.5 pmol)
We finally saw clear bands in all conditions. Unfortunately, BSA was responsible for the primary bands of lanes with thrombin. The smear for thrombin may be due to thrombin glycosylation. Because the fuzzy signal, it is difficult to tell if there is a shift when T5 is added to thrombin. Streptavidin does not appear to be binding S5. The fuzzy signal may be due to excessive protein concentrations; we shall try everything again at 40 pmol DNA + 20 pmol protein.
This time around, we could see new bands for S5. So what's the band consistent across the DNA lanes?
Lower concentration gel
We reconstituted Bovine thrombin in PBS this time around. Lanes are as follows:
Protein
1: SeeBlue Plus 2 ladder
2: thrombin (20 pmol)
3: thrombin (20 pmol) + T5 (40 pmol)
4: streptavidin (20 pmol)
5: streptavidin (20 pmol) + S5 (40 pmol)
6: streptavidin (20 pmol) + biotinylated oligos (40 pmol)
7: 1kb dna ladder
8: nothing + dye
9: T5 (40 pmol)
10: T5 (40 pmol) + thrombin (20 pmol)
11: S5 (40 pmol)
12: S5 (40 pmol) + streptavidin (20 pmol)
Conclusions:
1) Weird DNA band is not due to dye.
2) streptavidin-thrombin binding not detected.
3) Thrombin binding not detected.
Next: Primary: 1) Denature DNA before incubation.
2) Use human thrombin instead of bovine thrombin. 85% homology isn't enough.
Contingency plan: 1) Try a more sensitive binding assay.
2) Try S0, T0 instead of S5, T5
3) Try human thrombin.
4) Run a gel a shorter time to see if T5, biotinylated oligos are resolved separately from mystery band.
Week 7
7/24
This time around, we're denaturing the aptamers before incubating with protein. 5 minutes at 90 degrees followed by 5 minutes at 4 degrees. Unfortunately we haven't gotten human thrombin yet. We'll also try Silver staining, which is supposed to be more sensitive than Coomassie and EtBr.
Protein
1: SeeBlue Plus 2 ladder
2: Benchmark ladder
3: thrombin (20 pmol)
4: thrombin (20 pmol) + T5 (40 pmol)
5: streptavidin (20 pmol)
6: streptavidin (20 pmol) + S5 (40 pmol)
7: streptavidin (20 pmol) + biotinylated oligos (40 pmol)
8: 1kb dna ladder
9: T5 (40 pmol)
10: T5 (40 pmol) + thrombin (20 pmol)
11: S5 (40 pmol)
12: S5 (40 pmol) + streptavidin (20 pmol)
Note: I purposely stained longer than I should because I was determined to see streptavidin. It's there, (lane 7), but it doesn't show up on its own for some reason. The large blotch in the middle of all of this is annoying, too. I'm not sure of its origin. Note for silver stainers: staining for a long time (more than 10 minutes) causes the top and bottom edges of the gels to discolor, possibly making it difficult to resolve bands.
7/25
Low Mass Ladder [invitrogen]: 6 blunt-ended fragments from 100bp to 2000 bp Lambda DNA HindIII (lambda digested by HindIII) [neb]: 23130, 94116, ..., 125 bp
Morning: the big human thrombin experiment.
8% gel run at 120V for 1.5 hours.
lane 1: ladder
lane 2: human thrombin
lane 3: human thrombin + T5
lane 4: human thrombin + denatured T5
lane 5: bovine thrombin
lane 6: bovine thrombin + T5
lane 7: bovine thrombin + denatured T5
lane 8: ladder
lane 9: T5
lane 10: denatured T5
lane 11: denatured T5+ bovine
lane 12: denatured T5+ human
I also attempted to see if the DNA components of our aptamers were capable of binding each other. Each lane contains 40 pmol of oligo or complex. Ran a 12% gel for 1 hour at 120V. Incubation was for 15 minutes total. Denaturation: oligos mixed, heated to 90 C for 5 minutes, then rapidly cooled to 4 C for 5 minutes, followed by 5 minutes at room temperature.
Lane 1: ladder
Lane 2: T20
Lane 3: S20
Lane 4: T20 + S20
Lane 5: T20 + S20; denatured during incubation
Lane 6: T35
Lane 7: S35
Lane 8: T35+S35
Lane 9: T35 + S35; denatured during incubation
Lane 10: T50
Lane 11: S50
Lane 12: T50 + S50 (denaturation condition)
The complementary sections appeared to have come together at a pretty high rate, both for denaturing and non-denaturing conditions.
7/26
Human thrombin info:
T6884-1KU 035K7580
[Link to product info page (includes information sheet)]
1670 NIH units; 3093 NIH units/mg
In light of yesterday's disappointing results, today was an information gathering day. We got in contact with David Liu, who developed the streptavidin aptamer. He and his grad student, Thomas Snyder, have not tried gel shift assays and believe that other methods such as binding the proteins to a nitrocellulose membrane or using a Biacore are more reliable.
We also tried out mfold to determine the secondary structure of our aptamers. The streptavidin aptamers leave the binding region secondary structure intact. However, the thrombin aptamers bind to themselves in the binding region, which could have well explained the lack of binding we observed. Nevertheless, such problems should have been solved by denaturation. Images to come.
7/27
We now have three assays to try:
1) millipore nitrocellulose membranes
The protocol is described [|here]. We may learn how to use this from the good people of Liu lab.
2) Biacore 3000 at the Bauer Center for Genomics
A complex piece of machinery is able to detect how well substrates are binding to a surface (to which the protein in question is bound). We spoke to Clair Reardon of the Bauer Center today; she will soon send us information about using it.
3) Streptavidin beads
Happily, the DNA aptamers to streptavidin were developed to bind streptavidin conjugated to agarose beads. The hope is that we add aptamer, wash, elute, and hopefully we will see aptamer. Hopefully.
We tried adding thrombin to a protein membrane and seeing if we can visualize DNA with EtBr. The answer is.. no. Well sort of.
Dots are just above where I added solution.
First 3 rows of dots have thrombin added to them; last row is blanks
DNA was added to all dots. For rows 1,2,4: T5 added to column 1, 2, 3; S5 added to column 4, 5. For row 3, T20+S20 added except for column 1, where T5 was added. (this was a pretty informalexperiment)
Although there are slightly lighter spots under the transUV in the first few lanes, there is no distinction for the type of aptamer which is added. Furthermore, the UV light might just be highlighting certain areas that had more solution added to them instead of EtBr. Regardless, you can't see much.
In light of the secondary structure complications of the thrombin aptamer, I mixed together T20 and S20 to see if the complex, which no longer has the unfortunate binding site concealment, could bind to thrombin.
Lane 1: ladder
Lane 2: T20
Lane 3: T20 + thrombin
Lane 4: S20
Lane 5: S20 + thrombin
Lane 6: T20+S20
Lane 7: T20+S20+ thrombin
Oddly enough, we saw no gel shift for T20+S20, which we should have seen as demonstrated previously. While it is unclear that our adaptamer had actually formed, what is clear is that thrombin remained unbound.
Week 8
7/31
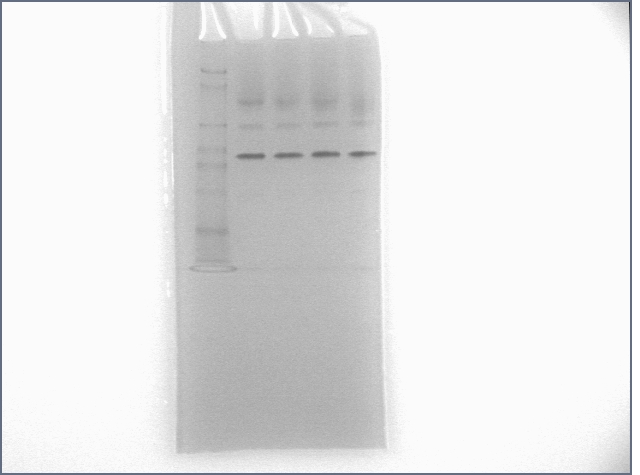
Ran pure thrombin aptamer sequence in a gel along with some friends for one hour. Results below.
Lanes:
1: protein ladder
2: thrombin
3: thrombin + T0
4: thrombin + T5
5: thrombin + T35+S35
6: DNA ladder
7: T0
8: T0 + thrombin
9: T5
10: T5 + thrombin
11: T35+S35
12: T35+S35 + thrombin
Protein: 20 pmol; DNA: 40 pmol. T35+S35 incubation: 30 minutes before addition of thrombin. 12% Polyacrylamide gel run for 1 hour at 120V.
If you compare lanes 2 with 5 and 11 with 12, you will notice a gel shift. If you were here, you'd notice me doing a little dance. :) :) :)
I should curb my enthusiasm a bit and note that the major thrombin band did not shift. Alain suggested that this might be a reason that papers tend to label the DNA, not the protein.
T35+S35 must be heavy enough to not run too far away while the aptamer region is coming off thrombin, unlike T0 (the pure thrombin aptamer). Running the gel for 1 hour may have made a difference, too. Maybe we'll see an even better shift using T50+S50.