IGEM:Harvard/2006/Adaptamers/Notebook/2006-8-15
7/15
Magnetic rack, more thrombin beads, and most importantlyT20, S20, S35, T50, S50, and A15 arrived today. Hurrah.
- Polystyrene beads
Tried out coupling AMF to polystyrene beads. Protocol given by Dr. Shih:
Generate a fresh aliquot of 0.2M EDC (dissolve 10 mg at a time).
Positive control: 10 uL unwashed optilink (non-magnetic) beads (10% suspension) + 10 uL 1 M MES pH 6.0 + 100 uL 100 uM aminomethylfluorescein + 20 uL 0.2M EDC (freshly dissolved)
Negative control: 10 uL unwashed optilink (non-magnetic) beads (10% suspension) + 10 uL 1 M MES pH 6.0 + 100 uL 100 uM aminomethylfluorescein + 20 uL water
Incubate 15 min at room temp. Spin in microfuge 15000 rcf 1 min. Remove the rest of the supernatant carefully, discard. Resuspend pellet in 100 uL 0.1M HEPES pH 7.5. Record fluorescence of the pellets on UV box using digital camera (same setup used to take pictures of ethidium bromide stained gels).
This took longer than expected due to our confusing pH meter and finding the necessary reagents, which will be added to the solutions page. Come to me for these if you need them.
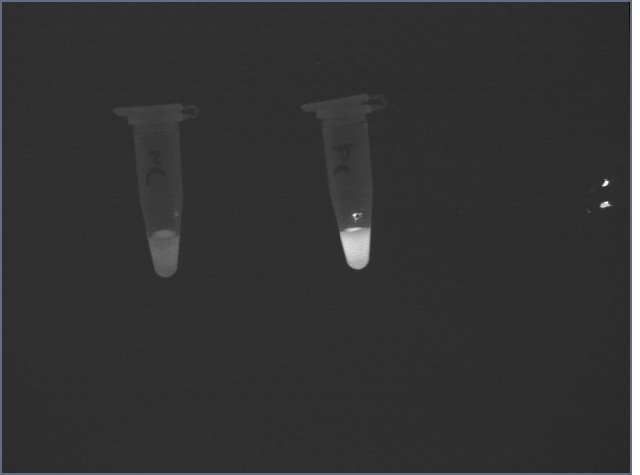
Following the suspension, the signal from the negative control was still high:
However, after two 1mL washes with Hepes buffer, the difference became clear:
- Coupling of thrombin to polystyrene beads
While a variety of procedures exist, I replicated Dr. Shih's procedure except using 250 uL 40 uM thrombin. This will just be an initial test run to see how much the most basic procedure yields.
- Testing of binding to thrombin-polystyrene beads.
The newly-coupled beads were tested for aptamer-binding.
5 uL coupled beads (total volume produced was 10 uL) were diluted to 10 uL and incubated either with 160 pmol thrombin aptamer or 160 pmol streptavidin aptamer. 5 uL of uncoupled beads were also incubated with 160 pmol thrombin aptamer for 30 minutes. Beads were then washed and eluted with 75 uL 26.7 uM thrombin. Elutions and washes were then run on a 4-20% PA gel in 1X TBE for 30 minutes and visualized by staining with EtBr.
No DNA was visible in washes with T0; the problem is likely that EtBr does not stain ssDNA well; we will try to stain with SYBR gold tomorrow.