IGEM:Harvard/2006/Cyanobacteria: Difference between revisions
JeffreyLau (talk | contribs) |
|||
| (21 intermediate revisions by 4 users not shown) | |||
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
Welcome to the lab notebook for the Cyanobacteria project! The goal of our team, composed of four members, is to reconstruct the cyanobacterial circadian oscillator system into E. coli. Three proteins, KaiA, B, and C, have been shown to have an in-vitro phosphorylation state oscillation (Nakajima et al. 2005) by transcriptional-translational independent methods. If this system can be reconstituted in E. coli, there are two important applications: | Welcome to the lab notebook for the Cyanobacteria oscillator project! The goal of our team, composed of four members, is to reconstruct the cyanobacterial circadian oscillator system into E. coli. Three proteins, KaiA, B, and C, have been shown to have an in-vitro phosphorylation state oscillation (Nakajima et al. 2005) by transcriptional-translational independent methods. If this system can be reconstituted in ''E. coli'', there are two important applications: | ||
#'''Synthetic Biology''': Creating a functional, oscillating set of proteins is the next logical step from the synthetic "repressilator" system engineered by Elowitz et al. (2000). Although a good proof of concept, the "repressilator" lacks the stability needed from a robust oscillator such as the naturally evolved cyanobacterial oscillator. This robust oscillator could prove useful in an eventual biocircuit. | #'''Synthetic Biology''': Creating a functional, oscillating set of proteins is the next logical step from the synthetic "repressilator" system engineered by Elowitz et al. (2000). Although a good proof of concept, the "repressilator" lacks the stability needed from a robust oscillator such as the naturally evolved cyanobacterial oscillator. This robust oscillator could prove useful in an eventual biocircuit. | ||
#'''Circadian Biology''': Cyanobacteria are the simplest model organisms for the study of circadian oscillation. Although circadian oscillation has been fairly well characterized, less is understood at the molecular level. By porting the oscillation system into E. coli, one can begin to understand more precisely the pathways involved in the genomic oscillation of cyanobacteria. | #'''Circadian Biology''': Cyanobacteria are the simplest model organisms for the study of circadian oscillation. Although circadian oscillation has been fairly well characterized, less is understood at the molecular level. By porting the oscillation system into ''E. coli'', one can begin to understand more precisely the pathways involved in the genomic oscillation of cyanobacteria. | ||
For more background information on the ciracadian system, please check out our "Literature" section. Otherwise, day-to-day work can be found under the "Lab Notebook" tab; we will post major results of our work and links to the days as they become available. If you have questions or comments, feel free to contact us: information is located at the main Harvard iGEM 2006 page. Thanks! | For more background information on the ciracadian system, please check out our "Literature" section. Otherwise, day-to-day work can be found under the "Lab Notebook" tab; we will post major results of our work and links to the days as they become available. If you have questions or comments, feel free to contact us: information is located at the main Harvard iGEM 2006 page. Thanks! | ||
| Line 15: | Line 15: | ||
Zhipeng, Hetmann, Dave, and Jeff | Zhipeng, Hetmann, Dave, and Jeff | ||
==Outline of Findings== | |||
*07/05/06: The incubator for growing up our cyanobacteria is complete; we have cultures growing! | '''Update 10/27/06:''' We believe we can express the three proteins into e. coli, and that there is interaction between A+C and possible interaction between B+C. See the Lab Notebook for more information. | ||
*07/10/06: Some computer modeling has been done to see the effect of multiple unsyncronized clocks on phosphorylation state output. | [[Image:102706_cyanoresult.jpg]] | ||
**[[/IGEM:Harvard/2006/Cyanobacteria/Notebook/2006- | |||
*10/25/06: Constructs for Stage I have been completed; ready to move to Stage I of Western Blotting, to verify expression of KaiC and interaction of KaiA and KaiB with KaiC. | ==Outline of Findings and Signifigant Dates== | ||
*07/05/06: The incubator for growing up our cyanobacteria is complete; we have cultures growing! [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-7-5 | Link]] | |||
*10/27/06: Preliminary data indicates that the Kai proteins are being expressed in e. coli and that there is interaction between the three proteins! | *07/10/06: Some computer modeling has been done to see the effect of multiple unsyncronized clocks on phosphorylation state output. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-7-10 | Link]] | ||
*07/21/06: Upon having trouble with site-specific mutagenesis on the KaiA and KaiBC operons from the cyanobacterial genome, we have decided to pursue synthesis of the constructs in parallel with continued extraction attempts. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-7-21 | Link]] | |||
*08/01/06: Preliminary success with site-specific mutagenesis. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-8-1 | Link]] | |||
*08/05/06: Promoter leakness tests come out negative. May have to use low-copy plasmids if we want good control of protein expression in Top10F. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-8-5 | Link]] | |||
*08/11/06: We are moving to the synthetic KaiA, KaiB, and KaiC for future work. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-8-11 | Link]] | |||
*08/30/06: We successfully made the first construct, Lac+RBS+KaiC. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-8-30 | Link]] | |||
*09/01/06: Using the newly developed ligation protocol, we have successfully repeated Lac+RBS+KaiC from 08/30/06 and made Lac+RBS+KaiA. [[IGEM:Harvard/2006/Nicholas_Stroustrup%27s_Notebook#Results_Summary | | |||
Link]] | |||
*10/21/06: Successfully made Lac+RBS+KaiB and Lac+RBS+KaiA+Lac+RBS+KaiC. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-10-21 | Link]] | |||
*10/24/06: Successfully made Lac+RBS+KaiB+Lac+RBS+KaiC. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-10-24 | Link]] | |||
*10/25/06: Constructs for Stage I have been completed; ready to move to Stage I of Western Blotting, to verify expression of KaiC and interaction of KaiA and KaiB with KaiC. [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-10-24 | Link]] | |||
*'''10/27/06: Preliminary data indicates that the Kai proteins are being expressed in e. coli and that there is interaction between the three proteins! [[IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-10-27 | Link]]''' | |||
==Construct Planning== | ==Construct Planning== | ||
| Line 37: | Line 47: | ||
* KaiC + J04500: 2110 bp | * KaiC + J04500: 2110 bp | ||
==Agenda== | ==Agenda== | ||
''See image at right for our long-term project outline.'' | ''See image at right for our long-term project outline.'' | ||
[[Image:Cyanobacteria_Flowchart.png|thumb|Long-term project outline]] | [[Image:Cyanobacteria_Flowchart.png|thumb|Long-term project outline]] | ||
==BioBricks Used== | ==BioBricks Used== | ||
| Line 90: | Line 88: | ||
*''[[Media:final_presentation_draft2.ppt |Final Presentation (complete)]]'' --old | *''[[Media:final_presentation_draft2.ppt |Final Presentation (complete)]]'' --old | ||
*[[:Image:Cyano presentation.ppt | Jamboree presentation]] (in progress) | *[[:Image:Cyano presentation.ppt | Jamboree presentation]] (in progress) | ||
**[[:Image:Cyano_presentation_script.doc|Script]] (in progress) | |||
*[[:Image:Cyano poster.ppt | Cyano poster]] (in progress) | |||
==Team Members== | ==Team Members== | ||
Latest revision as of 04:28, 3 November 2006
<html><style type='text/css'> .tabs {
font-size:80%;
font-weight:none;
width: 100%;
color: #FFFFFF;
background:#FFFFFF url("/images/5/54/DarkgreenTab-bg.gif") repeat-x bottom;
}
.tabs li {
background:url("/images/3/36/DarkgeenTab-left.gif") no-repeat left top;
}
.tabs a,.tabs strong {
background:url("/images/d/d3/DarkgreenTab-right.gif") no-repeat right top;
color:#FFFFFF;
padding: 3px 10px 3px 4px;
}
.tabs strong{
color:#CCFF00;
background-image:url("/images/b/b1/DarkgreenTab-right_on.gif");
}
.tabs a:hover{
color:#66FF00;
}
</style></html>
Introduction
Welcome to the lab notebook for the Cyanobacteria oscillator project! The goal of our team, composed of four members, is to reconstruct the cyanobacterial circadian oscillator system into E. coli. Three proteins, KaiA, B, and C, have been shown to have an in-vitro phosphorylation state oscillation (Nakajima et al. 2005) by transcriptional-translational independent methods. If this system can be reconstituted in E. coli, there are two important applications:
- Synthetic Biology: Creating a functional, oscillating set of proteins is the next logical step from the synthetic "repressilator" system engineered by Elowitz et al. (2000). Although a good proof of concept, the "repressilator" lacks the stability needed from a robust oscillator such as the naturally evolved cyanobacterial oscillator. This robust oscillator could prove useful in an eventual biocircuit.
- Circadian Biology: Cyanobacteria are the simplest model organisms for the study of circadian oscillation. Although circadian oscillation has been fairly well characterized, less is understood at the molecular level. By porting the oscillation system into E. coli, one can begin to understand more precisely the pathways involved in the genomic oscillation of cyanobacteria.
For more background information on the ciracadian system, please check out our "Literature" section. Otherwise, day-to-day work can be found under the "Lab Notebook" tab; we will post major results of our work and links to the days as they become available. If you have questions or comments, feel free to contact us: information is located at the main Harvard iGEM 2006 page. Thanks!
Sincerely,
Zhipeng, Hetmann, Dave, and Jeff
Update 10/27/06: We believe we can express the three proteins into e. coli, and that there is interaction between A+C and possible interaction between B+C. See the Lab Notebook for more information.
Outline of Findings and Signifigant Dates
- 07/05/06: The incubator for growing up our cyanobacteria is complete; we have cultures growing! Link
- 07/10/06: Some computer modeling has been done to see the effect of multiple unsyncronized clocks on phosphorylation state output. Link
- 07/21/06: Upon having trouble with site-specific mutagenesis on the KaiA and KaiBC operons from the cyanobacterial genome, we have decided to pursue synthesis of the constructs in parallel with continued extraction attempts. Link
- 08/01/06: Preliminary success with site-specific mutagenesis. Link
- 08/05/06: Promoter leakness tests come out negative. May have to use low-copy plasmids if we want good control of protein expression in Top10F. Link
- 08/11/06: We are moving to the synthetic KaiA, KaiB, and KaiC for future work. Link
- 08/30/06: We successfully made the first construct, Lac+RBS+KaiC. Link
- 09/01/06: Using the newly developed ligation protocol, we have successfully repeated Lac+RBS+KaiC from 08/30/06 and made Lac+RBS+KaiA. Link
- 10/21/06: Successfully made Lac+RBS+KaiB and Lac+RBS+KaiA+Lac+RBS+KaiC. Link
- 10/24/06: Successfully made Lac+RBS+KaiB+Lac+RBS+KaiC. Link
- 10/25/06: Constructs for Stage I have been completed; ready to move to Stage I of Western Blotting, to verify expression of KaiC and interaction of KaiA and KaiB with KaiC. Link
- 10/27/06: Preliminary data indicates that the Kai proteins are being expressed in e. coli and that there is interaction between the three proteins! Link
Construct Planning
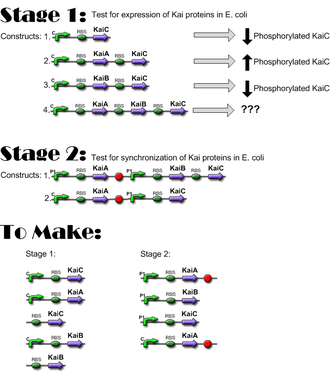
Lengths
From VF2 to VR (BioBrick primers):
- KaiA + J04500: 1406 bp
- KaiB + J04500: 859 bp
- KaiC + J04500: 2110 bp
Agenda
See image at right for our long-term project outline.

BioBricks Used
- <bbpart>BBa_J04450</bbpart>
- RFP device
- Insert size: 1069bp
- [pSB1A2]
- High-copy, AmpR
- Size: 2079bp
- <bbpart>BBa_J04500</bbpart>
- Lac promoter + RBS
- Insert size: 220bp
- [pSB1AK3]
- High-copy, AmpR, KanR
- Insert size: 3189bp
- [pSB4A3]
- Low-copy, AmpR
- Insert size: 3339 bp
- <bbpart>BBa_R0010</bbpart> + <bbpart>BBa_E0241</bbpart>
- GFP device
- Insert size: 995 bp
- <bbpart>BBa_J04450</bbpart>
Presentations
- Project proposal (week 2)
- Week 3 progress update
- Built incubator and obtained WH8102, PCC7942, and PCC6803 strains
- Week 4 progress update
- Week 5 progress update, upd. 10:10 7/17
- Week 6 progress update, upd. 10:02 7/24 HH
- Week 7 progress update
- Week 8 progress update
- Week 9 progress update
- Week 10 progress update, 50% complete
- Final Presentation (incomplete) --old
- Final Presentation (complete) --old
- Jamboree presentation (in progress)
- Script (in progress)
- Cyano poster (in progress)
Team Members
- Hetmann Hsieh (talk, edits)
- Jeffrey Lau (talk, edits)
- Zhipeng Sun (talk, edits)
- David Ramos (talk, edits)
Recent Changes
- N
- This edit created a new page (also see list of new pages)
- m
- This is a minor edit
- b
- This edit was performed by a bot
- (±123)
- The page size changed by this number of bytes
15 April 2024
|
|
23:43 | User:Yanbin Huang 2 changes history +170 [Yanbin Huang (2×)] | |||
|
|
23:43 (cur | prev) 0 Yanbin Huang talk contribs (→Granted Patents) | ||||
|
|
23:43 (cur | prev) +170 Yanbin Huang talk contribs (→Granted Patents) | ||||
|
|
22:11 | The paper that launched microfluidics - Xi Ning 14 changes history +9,705 [Xning098 (14×)] | |||
|
|
22:11 (cur | prev) −6 Xning098 talk contribs (→Summary) | ||||
|
|
22:07 (cur | prev) −12 Xning098 talk contribs (→Synthesis) | ||||
|
|
22:06 (cur | prev) 0 Xning098 talk contribs | ||||
|
|
22:06 (cur | prev) +1 Xning098 talk contribs | ||||
|
|
22:05 (cur | prev) 0 Xning098 talk contribs | ||||
|
|
22:03 (cur | prev) +630 Xning098 talk contribs | ||||
|
|
22:01 (cur | prev) +3,189 Xning098 talk contribs | ||||
|
|
21:44 (cur | prev) +688 Xning098 talk contribs (→Separation and quantification) | ||||
|
|
21:33 (cur | prev) +306 Xning098 talk contribs | ||||
|
|
21:29 (cur | prev) −2 Xning098 talk contribs (→Electrokinetic effect) | ||||
|
|
21:28 (cur | prev) −1 Xning098 talk contribs (→Separation and quantification) | ||||
|
|
21:27 (cur | prev) +398 Xning098 talk contribs (→Separation and quantification) | ||||
|
|
21:24 (cur | prev) +2,812 Xning098 talk contribs | ||||
|
|
21:06 (cur | prev) +1,702 Xning098 talk contribs | ||||
|
|
21:45 | (Upload log) [Xning098 (4×)] | |||
|
|
21:45 Xning098 talk contribs uploaded File:Figure 4 Tdesign.png | ||||
|
|
21:30 Xning098 talk contribs uploaded File:Figure 3 Set-up3.png | ||||
|
|
21:24 Xning098 talk contribs uploaded File:Figure 2 Set-up1.png | ||||
|
|
21:09 Xning098 talk contribs uploaded File:Figure 1 electroosmotic flow.png | ||||
| 17:02 | CHEM-ENG590E:Wiki Textbook diffhist +54 Mredder talk contribs (→Chapter 7 - Fiber-based Microfluidics) | ||||
| m 07:22 | Paper Microfluidic Device for Archiving Breast Epithelial Cells diffhist +6 Sarah L. Perry talk contribs | ||||
| 06:39 | Hu diffhist +66 Hugangqing talk contribs | ||||
14 April 2024
|
|
19:57 | Microfluidic Gradient Generators - Greg Schneider 6 changes history +919 [Nmhatre (6×)] | |||
|
|
19:57 (cur | prev) +50 Nmhatre talk contribs (→Varying Flowrates and Asymmetrical Geometry) | ||||
|
|
19:57 (cur | prev) +473 Nmhatre talk contribs (→Varying Flowrates and Asymmetrical Geometry) | ||||
|
|
19:56 (cur | prev) −472 Nmhatre talk contribs (→Nonlinear Gradients) | ||||
|
|
19:55 (cur | prev) +766 Nmhatre talk contribs (→Methods For Creating Non-linear Gradients) | ||||
|
|
18:56 (cur | prev) +51 Nmhatre talk contribs (→Mathematical Model) | ||||
|
|
18:56 (cur | prev) +51 Nmhatre talk contribs (→Induced Charge Electro-Osmosis (ICEO)) | ||||
