IGEM:Harvard/2006/DNA nanostructures: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
(→Notes) |
(→Notes) |
||
| Line 73: | Line 73: | ||
* Macaya's and Bock's selection buffer: 20 mM Tris-acetate, pH 7.4, 140 mM NaCl, 5 mM KCl, 1 mM CaCl<sub>2</sub>, 1 mM MgCl<sub>2</sub> | * Macaya's and Bock's selection buffer: 20 mM Tris-acetate, pH 7.4, 140 mM NaCl, 5 mM KCl, 1 mM CaCl<sub>2</sub>, 1 mM MgCl<sub>2</sub> | ||
* Liu's incubation buffer: 40 mM Tris, 20 mM CH<sub>3</sub>COOH, 2mM EDTA, 12.5 mM (CH<sub>3</sub>COO)<sub>2</sub>Mg, pH 8.0 | * Liu's incubation buffer: 40 mM Tris, 20 mM CH<sub>3</sub>COOH, 2mM EDTA, 12.5 mM (CH<sub>3</sub>COO)<sub>2</sub>Mg, pH 8.0 | ||
* Liu's PAGE buffer: 1x TAE/Mg<sup>2+</sup> | |||
====Bibliography==== | ====Bibliography==== | ||
Revision as of 11:09, 10 July 2006
Project Overview
- Our goal is to to design and implement molecular containers, which can be dynamically opened and closed by an external stimulus.
- The containers will be implemented as DNA nanostructures, which afford a significant degree of positional control and chemical versatility.
- As an initial proof-of-concept, we plan to use our DNA containers to demonstrate controllable activation ("delivery") of anti-thrombin aptamers.
- We expect that molecular containers could have several interesting scientific and clinical applications, such as
- Drug and gene delivery
- Bio-marker scavenging (early detection of biomarkers)
- Directed evolution (compartmentalized selections)
- Using multiplexing for combinatorial chemical synthesis
- Capture and stabilization of multiprotein complexes
- Protein folding (chaperones)
- Cell sorting
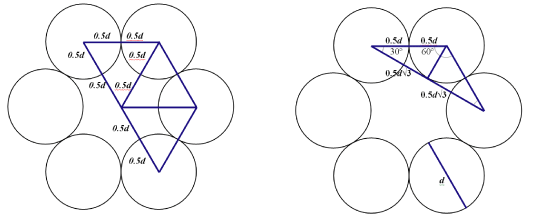
Container Specs
Container Designs
-
Design 1
hexagonal core, separate 1-ply lids -
Design 2
hexagonal core, separate 2-ply lids -
Design 3
rectangular core, continuous 1-ply lids -
Design 4
hexagonal core, separate 1-ply lids
Latch Designs
Coding
Existing code
Thrombin-aptamer experiments
Notes
Questions / procedures
- what percent gel? 10% to 20% polyacrylamide gels, no SDS (but would make for a good control)
- what incubation conditions?
- how much protein and DNA? protein at 1 μM, DNA at 2 μM
- Coomassie stain
Experiments
| number | thrombin | aptamer | nanotube | DNA-stained prediction | protein-stained prediction |
| 0 | - | - | - | no bands | no bands |
| 1 | - | - | + | slow band (nanotube) | no bands |
| 2 | - | + | - | fast band (aptamer) | no bands |
| 3 | - | + | + | slow band (aptamer-nanotube), traces of fast band (aptamer) | no bands |
| 4 | + | - | - | no bands | fast band (thrombin) |
| 5 | + | - | + | slow band (nanotube) | fast band (thrombin) |
| 6 | + | + | - | medium band (aptamer-thrombin), fast band (aptamer) | medium band (aptamer-thrombin), traces of fast band (thrombin) |
| 7 | + | + | + | very slow band (thrombin-aptamer-nanotube), slow band (aptamer-nantotube), traces of fast band (aptamer) | very slow band (thrombin-aptamer-nanotube), medium band (aptamer-thrombin), traces of fast band (thrombin) |
Buffers
- Macaya's and Bock's selection buffer: 20 mM Tris-acetate, pH 7.4, 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2
- Liu's incubation buffer: 40 mM Tris, 20 mM CH3COOH, 2mM EDTA, 12.5 mM (CH3COO)2Mg, pH 8.0
- Liu's PAGE buffer: 1x TAE/Mg2+
Bibliography
- Schultze P, Macaya RF, and Feigon J. Three-dimensional solution structure of the thrombin-binding DNA aptamer d(GGTTGGTGTGGTTGG). J Mol Biol. 1994 Feb 4;235(5):1532-47. DOI:10.1006/jmbi.1994.1105 |
- Liu Y, Lin C, Li H, and Yan H. Aptamer-directed self-assembly of protein arrays on a DNA nanostructure. Angew Chem Int Ed Engl. 2005 Jul 11;44(28):4333-8. DOI:10.1002/anie.200501089 |
- Li WX, Kaplan AV, Grant GW, Toole JJ, and Leung LL. A novel nucleotide-based thrombin inhibitor inhibits clot-bound thrombin and reduces arterial platelet thrombus formation. Blood. 1994 Feb 1;83(3):677-82.
- Bock LC, Griffin LC, Latham JA, Vermaas EH, and Toole JJ. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992 Feb 6;355(6360):564-6. DOI:10.1038/355564a0 |
- Macaya RF, Schultze P, Smith FW, Roe JA, and Feigon J. Thrombin-binding DNA aptamer forms a unimolecular quadruplex structure in solution. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3745-9. DOI:10.1073/pnas.90.8.3745 |
Presentations
Most recent (Week 3)
Week 2: Original proposal
Working Team Members
- Tiffany Chan (talk)
- Katherine Fifer (talk)
- Valerie Lau (talk)
- Matthew Meisel (talk)
- ...and others are welcome!