IGEM:Harvard/2008/Lab Notebooks/DailyBook/Week4/Chemical and Light: Difference between revisions
Meng Xiao He (talk | contribs) (→tttt) |
|||
| Line 818: | Line 818: | ||
=Test of IPTG Inducible System= | =Test of IPTG Inducible System= | ||
Revision as of 06:58, 21 July 2008
PCR/primers
HO+pcyA, P3 vector w/o GFP
Primers were designed to PCR out the heme oxygenase and pcyA coding regions from the UT Austin plasmids. Due to the RE sites in these nonBB plasmids, their ends are given ApaLI and KpnI sites. The same is done for the P3 vector (the primers are such that we can PCR out the backbone, along with the RBS and terminator. The "P3-GFP" primers should also work for P1. However, I'm a little concerned, b/c the annotation of the P1 and P3 vectors disagrees with the sequence given for the RBS and terminator subparts.
mtrB
The same thing was done for mtrB (as for HO+pcyA). This gives us constitutive mtrB for testing the complement.
7/17: PCR
The primers arrived today, so PCR was performed using a colony of Shewanella Δ EnvZ (which should still have mtrB).
Rx mix: 90μL PCR supermix, 2μL mtrB-ApaLI-F primer (20μM), 2μL mtrB-KpnI-R (20μM), swirl of colony
Rx: 10min @ 94°C → 10x[45s @ 94°C → 45s @ 52°C → 2m30s @72°C] → 20x[45s @ 94°C → 45s @ 54°C → 4m45s @72°C]→ 5x[45s @ 94°C → 45s @ 56°C → 4m45s @72°C]→5min @ 72°C → ∞ @ 4°C
PCR failed- perhaps annealing temperature was too high or too many cells. Will retry.
7/20: Retry (Gradient)
Rx mix (split into 12 samples): 180μL PCR supermix, 4μL mtrB-ApaLI-F primer (20μM), 4μL mtrB-KpnI-R (20μM), swirl of WT Shewanella colony, 12μL water
Rx: 5min @ 94°C → 35x[45s @ 94°C → 45s @ {43.0, 43.2, 43.8, 44.5, 45.5, 46.9, 48.4, 49.7, 50.6, 51.3, 51.8, 52.0}°C → 2m38s @72°C] → 5min @ 72°C → ∞ @ 4°C
P38, P39 Oligos
5' phospho oligos were made to anneal into what is effectively P38 and P39 digested with ES.
P11
7/16
P11 (a piece of the filter paper) was PCRed: 2μL EB, 45μL PCR supermix, 1μL BBpfx primer (20μM), 1μL BBsfx (20μM)
Rx: 5min @ 94°C → 15x[45s @ 94°C → 45s @ 54°C → 4m45s @72°C] → 20x[45s @ 94°C → 45s @ 56°C → 4m45s @72°C]→ 5min @ 72°C → ∞ @ 4°C
7/17
PCR failed- no 4kb band
CDF
8 100μL PCR reactions set up (standard mix): DNA template is S1 P13, 40 cycles, annealing temp is 58°C, extension time is 1:15
Run on gel; bands cut and frozen.

|
1.2% agarose E-gel run for 30 min and visualized using EtBr/UV | |
|---|---|---|
| Lane | Contents | |
| 1 | 1 KB ladder | |
| 2-4 | CDF (~900 bp) | |
| 5-12 | mtrB w/ BB ends (~2.1kb) | |
mtrB
8 100μL PCR reactions set up (standard colony mix) w/ WT Shewanella
Rx: 5min @ 94°C → 10x[45s @ 94°C → 45s @ 52°C → 2m45s @72°C] → 25x[45s @ 94°C → 45s @ 55°C → 2m38s @72°C]→ 5min @ 72°C → ∞ @ 4°C
Run on gel; bands cut and frozen.
Ligations
Results from 7/12 ligations (transformed into E1), 7/16 transformations
| DNA | Selection used on plate | # colonies | Notes | Liquid cultures of retransformed E1 (plating skipped) 7/16 |
| 17XP+38SP | AMP | 2 AFTER 48HRS | Plate put in 4°C, not picked | no growth |
| 63+59 FROM 7/9 LIGATION | KAN | 1 AFTER 48HRS | no GFP, so plate tossed | no growth |
| CDF XP (7/4)+P1XP | KAN | 1 AFTER 48HRS | has GFP- indicates wrong plasmid, tossed | -- |
| 45XP(7/11 45)+39SP | AMP | 0 | no growth | |
| 45XP(7/11 45)+38SP | AMP | 0 | no growth | |
| 77XP+38SP | AMP | 0 | no growth | |
| 52XP+39SP | AMP | 0 | no growth | |
| 77XP+39SP | AMP | 0 | no growth | |
| 89XP+38SP | AMP | 0 | no growth | |
| 45XP+39SP | AMP | 0 | no growth | |
| 89XP+39SP | AMP | 0 | no growth | |
| 51XP+39SP | AMP | 0 | no growth | |
| 17XP+39SP | AMP | 0 | no growth | |
| P88 | KAN | 0 | -- | |
| 63+58 FROM 7/9 LIGATION | KAN | 0 | no growth | |
| 58EX+63ES | KAN | 0 | no growth | |
| 52XP+38SP | AMP | 0 | no growth | |
| 89ES+45EX | CARB | TMTC | The 45 EX used was not purified, so the miniprep culture was of religated P45 and did not glow. However, we picked fluorescent colonies from the plate and restreaked/set up liquid cultures of them. 7/15: Pelleted impure cultures were streaked for isolation. Individual fluorescent colonies picked for miniprep cultures (7/16: did not fluoresce, plates tosses). | -- |
| CDF XP(7/4)+P1XP(7/1) | KAN | 68 | miniprepped | -- |
| 17ES+45EX | CARB | TMTC | The 45 EX used was not purified, so the miniprep cultures were of religated P45 and did not glow. However, we picked fluorescent colonies from the plate and restreaked/set up liquid cultures of them. 7/15: Miniprep done, glycerol stock made, pelleted impure cultures were streaked for isolation (7/16: did not fluoresce, plates tosses). Individual fluorescent colonies picked for miniprep cultures. | -- |
| 45XP+38SP | AMP | 4 | DID NOT FLUORESCE- not miniprepped | grew, streaked for colonies- no GFP, tossed |
| 51XP+38SP | CARB | 5 | 2 picked colonies, neither grew- repicked 7/14, these did not grow either | no growth |
| 54ES+59EX | KAN | 3 | DID NOT FLUORESCE- not miniprepped, no GFP, so plate tossed | no growth |
| CDF XP(7/12)+P1XP(7/1) | KAN | TMTC | miniprepped | -- |
| CDF XP(7/12)+P1 | KAN | 232 | miniprepped | -- |
| 77ES+59EX | KAN | NOT PLATED | no growth | |
| no DNA | AMP | 0 | -- | |
| no DNA | KAN | 0 | -- | |
| no DNA | CARB | 6 big + 10 small | -- | |
| no cells | KAN | 0 | no growth | |
| 1μL pUC19 | CARB | 96 | did grow in LB AMP medium (+ control) | -- |
| 1μL pUC19 | AMP | 64 | -- |
We retransformed all of these on 07/15 using 5 μL DNA for 50 μL DH5α cells. We put the cells directly into liquid cultures after transforming them.
RE digests 07/15
We digested P53/54 with ES and P58/59 with EX. We intend to ligate these parts together. This will give us RBS + TetR/LacI + terminator + pTet/pLac + RBS + GFP + terminator.
We also digested P90 with SP and P48/P49 with XP. This will give us the CDF origin in a p15A vector with an Amp or Cm resistance cassette.
We followed the standard digest protocol under the "General Protocols" section.
Gel 1
Lane 1: 1 kb ladder
Lane 2: P48 cut XP (769 bp)
Lane 3: P48 uncut (3477 bp)
Lane 4: P49A cut XP (943 bp)
Lane 5: P49A uncut (3651 bp)
Lane 6: P49B cut XP (943 bp)
Lane 7: P49B uncut (3651 bp)
Lane 8: P49C cut XP (943 bp)
Lane 9: P49C uncut (3651 bp)
Lane 10: P53 cut ES (840 bp)
Gel 2
Lane 1: P53 uncut (2919 bp)
Lane 2: P54 cut ES (1308 bp)
Lane 3: P54 uncut (3387 bp)
Lane 4: P58 cut EX (~3016 bp)
Lane 5: P58 uncut (3016 bp)
Lane 6: P59A cut EX (~3201 bp)
Lane 7: P59A uncut (3201 bp)
Lane 8: P59B cut EX (~3201 bp)
Lane 9: 1 kb ladder
Gel 3
Lane 1: P59B uncut (3201 bp)
Lane 2: P59C cut EX (~3201 bp)
Lane 3: P59C uncut (3201 bp)
Lane 4: P90A cut SP (~3650 bp)
Lane 5: P90A uncut (~3650 bp)
Lane 6: P90A cut SP (~3650 bp)
Lane 7: P90A uncut (~3650 bp)
Lane 8: P90A cut SP (~3650 bp)
Lane 9: P90A uncut (~3650 bp)
Lane 10: blank
Lane 11: 1 kb ladder
RE digests 07/16
We digested
- P63, P76 (A, B, C, D), P91 (A, B, C) with EX
- P90 (α, β) with SP
- P48, P49 (A, B, C) with XP
We used the standard digestion protocol.
We plan to ligate
- P90 with P48/P49. This will give us the CDF origin in the P1 vector with Amp and Cm resistance cassettes. We will also add P63, a terminator, and the high and low constitutive promoters (P38 and P39) later.
- P91/P76 with the high and low constitutive promoters. This should give us the entire Lac and Tet plasmids.
Gel 1
- 1 kb ladder
- P48 cut XP
- P48 uncut
- P49A cut XP
- P49A uncut
- P49B cut XP
- P49B uncut
- P49C cut XP
- P49C uncut
- P63 cut EX
- P63 uncut
- P76A cut EX
- P76A uncut
- P76B cut EX
- P76B uncut
Gel 2
- 1 kb ladder
- P90α cut SP
- P90α uncut
- P90β cut SP
- P90β uncut
- P91A cut EX
- P91A uncut
- P91B cut EX
- P19B uncut
We extracted and purified P48, P49A, P49B, P63
Ligations 07/15
We ligated
- P90 SP with P48 XP
- P90 SP with P49 XP
- P58 EX with P53 ES
- P59 EX with P53 ES
These ligations were transformed into TOP10 cells that we made chemically competent.
Results 7/16
Transformed in E2:
| ' | Selection | # colonies |
| P90 SP with P48 XP | KAN | 0 |
| P90 SP with P49 XP | KAN | 1 - set up liquid culture |
| P58 EX with P53 ES | KAN | 0 |
| P59 EX with P53 ES | KAN | 0 |
| NO DNA | KAN | 0 |
Cross Transformations in S1
We transformed cells with repressor plasmid (P27), and duet vector (P12 and P13) with LacI with p59 (pLac + GFP) and vice versa in order to test the inducible system in S1.
Transformations (7/11) and Picked Colonies (7/13)
| Plate | Marker | Description | Picked Colonies? |
| S1 P34 | Amp | Lawn, restreaked. Many orange colonies along streak. Can\'t detect fluor. | Yes |
| S1 P59b cells + P12 vector | Amp | Lawn, restreaked. Fluorescent. ? | Yes |
| S1 P13 cells + P59b vector (1) | Kan | Many small colonies-- some fluoresce while others don\'t. Pinkish centers. | Yes (both F and no F) |
| S1 P13 cells + P59b vector (2) | Kan | More colonies than (1), but also some fluoresce while others don\'t. Pinkish centers. | Yes (both F and no F) |
| S1 P27 cells + P59B vector (1) | Kan | Many small colonies - fluorescent. | Yes |
| S1 P27 cells + P59B vector (2) | Kan | Almost lawn of tiny colonies - fluorescent. | Yes |
| S1 P59b Cells + P13 vector | Sm | Medium # of medium sized colonies, pink centers, no fluorescence. | Yes |
Diluted and Re-grew in Media with Both Antibiotics 7/14
| Sample | Media (5mL of each) | Grew? |
| S1 P59b + P12 | Amp+Kan | |
| S1 P13 + P59b (1) | Kan+Sm | |
| S1 P13 + P59b (2) | Kan+Sm | |
| S1 P27 + P59b (1) | Kan+Sm | |
| S1 P27 + P59b (2) | Kan+Sm | |
| S1 P59b + P12 | Kan+Sm |
Restreaked in Plates with Double Selection Markers 7/15
| Sample | Plate (original w/ second antibiotic added on) | Grew? |
| S1 P59b + P12 | ||
| S1 P13 + P59b (1) | ||
| S1 P13 + P59b (2) | ||
| S1 P27 + P59b (1) | ||
| S1 P27 + P59b (2) | ||
| S1 P59b + P12 |
Testing Cultures with IPTG 7/15
Thermoinducible Lac System
Grew up cultures of P84 (Lac mut265) and P85 (Lac mut 241) according to protocol derived from [Chao et al. 2002] and [Yabuta et al. 1995]
Testing Thermoinducible Cultures 7/14
- Grow up 10mL culture overnight with antibiotic at 200 RPM, 37 degrees.
- Dilute 2.5mL of overnight culture into 50mL of fresh LB.
- Grow at 200 RPM, 30 degrees until OD660 = 0.2.
- Split culture into two 25mL cultures, and put one in 30 degrees and the other in 40 degrees.
- Grow for 4 hours at these separate temperatures at 200 RPM.
- OD and measure YFP fluorescence (Ex: 514, Em: 527).
Results from First Thermoinducible Test 7/14
Controls:
| Control | OD660 | YFP (514/527) | YFP/OD |
| LB | 0 | 36.23 | |
| E1 + pET-Duet Vector | 0.361 | 288.07 | 797.9778393 |
Results:
| Sample | Temperature | Measurement | 1 to 1 dilution | 1 to 2 dilution | 1 to 4 dilution | Undiluted |
| P84 | 30 | YFP | 689.21 | 643.31 | 372.8 | 1308.37 |
| OD | 0.821 | 0.698 | 0.369 | 1.582 | ||
| YFP/OD | 839.4762485 | 921.6475645 | 1010.298103 | 827.0353982 | ||
| Corrected for autofluor. | 41.49840914 | 123.6697251 | 212.3202636 | 29.05755889 | ||
| 40 | YFP | 792.73 | 607.11 | 412.16 | 1326.16 | |
| OD | 0.884 | 0.64 | 0.405 | 1.665 | ||
| YFP/OD | 896.7533937 | 948.609375 | 1017.679012 | 796.4924925 | ||
| Corrected for autofluor. | 98.77555433 | 150.6315357 | 219.701173 | -1.485346843 | ||
| P85 | 30 | YFP | 937.12 | 665.39 | 405.58 | 1341.37 |
| OD | 1.178 | 0.739 | 0.411 | 1.638 | ||
| YFP/OD | 795.5178268 | 900.3924222 | 986.8126521 | 818.9072039 | ||
| Corrected for autofluor. | -2.46001251 | 102.4145829 | 188.8348127 | 20.92936457 | ||
| 40 | YFP | 862.44 | 600.56 | 439.47 | 1364.59 | |
| OD | 1.003 | 0.633 | 0.44 | 1.711 | ||
| YFP/OD | 859.8604187 | 948.7519747 | 998.7954545 | 797.5394506 | ||
| Corrected for autofluor. | 61.88257941 | 150.7741354 | 200.8176152 | -0.438388722 |
Putting Thermoinducible Lac and pLambda + GFP System on p15a Vector
RE Digestion 7/11
| ' | P1 (vector) | P1 (vector) | P74 (insert) | P84 (insert) | P85 (insert) |
| DNA | 15 uL | 15 uL | 5 uL | 5 uL | 5 uL |
| 10X Buffer (Volume & #) | 2.5 uL of 3 | 2.5 uL of 3 | 2.5 uL of 3 | 2.5 uL of 3 | 2.5 uL of 3 |
| 100X BSA | 0.25 uL | 0.25 uL | 0.25 uL | 0.25 uL | 0.25 uL |
| Restriction Enzyme 1 | 1 uL XbaI | 1 uL XbaI | 1 uL XbaI | 1 uL XbaI | 1 uL XbaI |
| Restriction Enzyme 2 | 1 uL PstI | 1 uL PstI | 1 uL PstI | 1 uL PstI | 1 uL PstI |
| Water | 5.25 uL | 5.25 uL | 15.25 uL | 15.25 uL | 15.25 uL |
| Total Volume | 25 uL | 25 uL | 25 uL | 25 uL | 25 uL |
Gel 7/14

|
1% Agarose, visualized using EtBr/UV | |
|---|---|---|
| Lane | Sample | |
| 1 | 1 KB ladder | |
| 2 | P1, XP, 2750 bp | |
| 3 | P1, XP, 2750 bp | |
| 4 | P1 uncut | |
| 6 | P74, XP, ~925 bp | |
| 7 | P74, uncut | |
| 9 | P84, XP, 2314 bp | |
| 10 | P84, uncut | |
| 11 | P85, XP, 2314 bp | |
| 13 | P85, uncut | |
P1 and P74 cut and uncut bands are in the right place, but P84 and P85 both have 2079 bp backbones, which does not account for the ~900 bp bands. We searched the sequence of the backbone in the registry for internal cut sites that could have produced the band, but found none.
Only P1 and P74 were extracted and gel purified.
Using BioBricks Primers to PCR Amplify P84-5 7/14
| Sample | PCR Supermix | Template DNA | BBx Fwd | BBx Rev | Water (to 50 uL) |
| P84 | 45 uL | 1 uL | 1 uL | 1 uL | 2 uL |
| P85 | 45 uL | 1 uL | 1 uL | 1 uL | 2 uL |
| (-) ctrl, S1 P4 | 45 uL | 1 uL | 1 uL | 1 uL | 2 uL |
| (+) ctrl, E1, P3 | 45 uL | 1 uL | 1 uL | 1 uL | 2 uL |
Gel from PCR 7/15

|
1% Agarose, visualized using EtBr/UV | |
|---|---|---|
| Lane | Sample | |
| 1 | 1 kb Ladder | |
| 2 | P84, ~2314 bp | |
| 3 | Empty | |
| 4 | P85, ~2314 bp | |
| 5 | Empty | |
| 6 | (+) ctrl, E1 P3 | |
| 7 | Empty | |
| 8 | (-) ctrl, S1 P4 | |
| 9 | 100 bp Ladder | |
| 10 | Empty | |
P84 and P85 bands were extracted and gel purified so they could be digested with XP.
Digestion of PCR Products with XP 7/15
| ' | P84 (insert) | P85 (insert) |
| DNA | 5 uL | 5 uL |
| 10X Buffer (Volume & #) | 2.5 uL of 3 | 2.5 uL of 3 |
| 100X BSA | 0.25 uL | 0.25 uL |
| Restriction Enzyme 1 | 1 uL XbaI | 1 uL XbaI |
| Restriction Enzyme 2 | 1 uL PstI | 1 uL PstI |
| Water | 15.25 uL | 15.25 uL |
| Total Volume | 25 uL | 25 uL |
After digestion, samples were PCR purified (7/16).
Single and Triple Digests of P84-5 7/14
P84 and P85 were found to have an internal XmnI cut site, so single cut digests were performed for both samples with XbaI, PstI, and XmnI. Triple digests were also done with all three enzymes. Digestions were incubated overnight at 37°C.
Gel of Single and Triple Digests 7/15
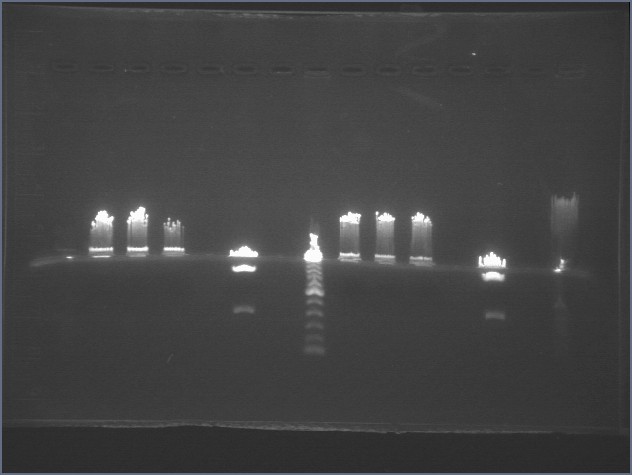
|
1% Agarose, visualized using EtBr/UV | |
|---|---|---|
| Lane | Sample | |
| 1 | 1 KB ladder | |
| 2 | P84, XbaI Single Cut | |
| 3 | P84, PstI Single Cut | |
| 4 | P84, XmnI Single Cut | |
| 5 | Empty | |
| 6 | P84, XPXmnI Triple Cut | |
| 7 | Empty | |
| 8 | 100 bp Ladder | |
| 9 | P85, XbaI Single Cut | |
| 10 | P85, PstI Single Cut | |
| 11 | P85, XmnI Single Cut | |
| 12 | Empty | |
| 13 | P85, XPXmnI Triple Cut | |
| 14 | Empty | |
| 15 | 1 KB ladder | |
The 2314 bp bands for the triple cuts of P84-5 were extracted and gel purified.
Ligation of P74, Triple Digested P84-5, and P1 7/15
Dephosphorylation of P1, 7/16
| ' | P1 |
| Vector | 15 uL |
| Dephos Buffer 10x | 2 uL |
| Alkaline Phosphatase | 1 uL |
| Water | 2 uL |
| Total | 20 uL |
Ligation 7/15
The P84-5 inserts are roughly the same size as the vector (P1) so we tried different ratios of insert to vector.
| ' | P1/P74 | P1/P84 1:3 | P1/P84 1:1 | P1/P85 1:3 | P1/P85 1:1 |
| Vector | 2 uL | 2 uL | 3 uL | 2 uL | 3 uL |
| Insert | 6 uL | 6 uL | 3 uL | 6 uL | 3 uL |
| DNA Dilution Buffer 5x | 2 uL | 2 uL | 2 uL | 2 uL | 2 uL |
| Water | 0 uL | 0 uL | 0 uL | 0 uL | 0 uL |
| (Added last:) | |||||
| T4 DNA Ligation Buffer 2x | 10 uL | 10 uL | 10 uL | 10 uL | 10 uL |
| T4 DNA Ligase | 1 uL | 1 uL | 1 uL | 1 uL | 1 uL |
| Total | 21 uL | 21 uL | 21 uL | 21 uL | 21 uL |
Ligations were then transformed in DH5α cells.
Transformation of P1/P74 and P1/P84-5 Triple Cuts 7/15
5 uL Ligation in 50 uL chemically competent E1 cells (old batch). Plates were incubated overnight at 37°C.
| Plate | Marker | # Colonies | Other Description |
| P75 (P1 + P74) | Kan | >100 | Small, white, fluorescent under microscope. |
| P92 (P1 + P84) 1:1 | Kan | 2 or 3 | Small, white |
| P92 (P1 + P84) 1:3 | Kan | 0 | |
| P93 (P1 + P85) 1:1 | Kan | 2 | Small, white |
| P93 (P1 + P85) 1:3 | Kan | 1 | Small, white |
| Kan ctrl (EB buffer) | Kan | 0 |
For positive control plates, see the Competent Cell Test Plates under Housekeeping.
Picked Colonies for Liquid Culture 7/16
All cultures grew, except for (-) ctrl.
Minipreps of Liquid Culture 7/17
| Plasmid | ng/uL | A260 | 260/280 | 260/230 | Constant |
| E1 P75a | 95.34 | 1.907 | 1.95 | 2.24 | 50 |
| E1 P75b | 108.59 | 2.172 | 1.98 | 2.21 | 50 |
| E1 P92a 1:1 | 126.61 | 2.532 | 2.01 | 1.67 | 50 |
| E1 P92b 1:1 | 129.67 | 2.593 | 1.98 | 2.09 | 50 |
| E1 P93a 1:1 | 164.37 | 3.287 | 1.94 | 1.95 | 50 |
| E1 P93b 1:1 | 106.08 | 2.122 | 1.92 | 2.14 | 50 |
| E1 P93 1:3 | 142.49 | 2.85 | 2.03 | 2.18 | 50 |
Ligation and Transformation of PCR Amplified P84-5 with P1 (7/16)
Ligation of P84-5 PCR with Dephos P1, 7/16
P1 was dephosphorylated the day before, and used for this ligation reaction as well.
| ' | P1/P84 PCR (1:3) | P1/P85 PCR (1:3) |
| Vector | 2 uL | 2 uL |
| Insert | 6 uL | 6 uL |
| DNA Dilution Buffer 5x | 2 uL | 2 uL |
| Water | 0 uL | 0 uL |
| (Added last:) | ||
| T4 DNA Ligation Buffer 2x | 10 uL | 10 uL |
| T4 DNA Ligase | 1 uL | 1 uL |
| Total | 21 uL | 21 uL |
Transformation of P92-3 PCR into E1, 7/16
5 uL Ligation in 50 uL chemically competent E1 cells (old batch). Plates were incubated overnight at 37°C.
| Plate | Marker | # Colonies |
| P92 PCR | Kan | 50+ small, white colonies. |
| P93 PCR | Kan | 50+ small white colonies |
| (+) ctrl pUC19 | Amp | >100 small white colonies |
| (-) ctrl | Kan | 0 colonies |
Liquid Cultures (7/17) and Minipreps (7/18)
| Plasmid/ Sample | ng/uL | A260 | 260/280 | 260/230 | Constant |
| E1 P92a PCR | 111.17 | 2.223 | 1.92 | 2.29 | 50 |
| E1 P92b PCR | 121.44 | 2.429 | 1.98 | 2.29 | 50 |
| E1 P93a PCR | 99.13 | 1.983 | 2.05 | 2.39 | 50 |
| E1 P93b PCR | 126.28 | 2.526 | 1.93 | 2.18 | 50 |
| S1 P59b 1 | 450.61 | 9.012 | 1.9 | 2.33 | 50 |
| S1 P59b 2 | 328.89 | 6.578 | 1.92 | 2.33 | 50 |
| E1 P58a 1 | 144.21 | 2.884 | 2 | 2.38 | 50 |
| E1 P58a 2 | 91.06 | 1.821 | 1.9 | 2.38 | 50 |
| E1 P80 | 128.63 | 2.573 | 2.04 | 2.26 | 50 |
Transformation of P75 and P92-3 (from 3x Cut) into S1 7/17
Used S1 cells frozen for electroporation.
| Plasmid/ Sample | Vol DNA Added (250-300 ng DNA) | Marker | Plate After O/N incubation at 30 (7/18) | Plate Description after TWO days incubation at 30 (7/19) |
| P75a | 3 uL | Kan | 2 white colonies, ~1 mm diam. Fluorescent | 4 pink/ yellow colonies (4mm), ~20 white/cream colonies, 1mm |
| P75b | 3 uL | Kan | 2 white colonies, ~1 mm diam. Fluorescent. | 4 pink/ yellow colonies (4mm), ~30 white/cream colonies, 1mm |
| P93 1:3 | 2 uL | Kan | ~6 white, 1mm colonies, dimmish under scope* | 8 pink/yellow colonies (4mm), ~120 white/cream colonies, 1mm |
| P58b (miniprepped from S1) | 5 uL | Kan | 0 colonies | ~250 white/ cream colonies, 1mm |
| (+) ctrl (S1 P59b) | 3 uL | Kan | >100 white, 1mm colonies | 120 pink/yellow colonies (4mm), ~10 white/ cream colonies, 1mm |
| (-) ctrl | None added | Kan | 0 colonies | ~20 white/ cream colonies, 1mm |
1mm white/ cream colonies present on all plates after the 2 day incubation resemble the contamination we have observed in other E. coli plates. This is of interest because Kan is not as vulnerable to degradation as Carb/ Amp are.
P75a and P75b were both very bright in S1. Thermoinducible cI857 was ordered from [ATCC].
The plates of P84-5 originally transformed in E. coli from the registry were used as a standard for fluorescence for the thermoinducible Lac colonies. They were left overnight at 37°C to stimulate thermosensitivity, which should lead to YFP expression. Although they were not very bright, they glowed. Compared to these plates, the P93 1:3 transformed in S1 was noticeably dimmer, though it had been incubated at 30°C O/N. Plate will be restreaked and grown at different temperatures after the weekend.
Transformation of P92-3 (from PCR) into S1 7/18
| Plasmid/ Sample | Vol DNA Added (250-300 ng DNA) | Marker | Plate After O/N incubation at 30 | Plate Description after TWO days incubation at 30 |
| P92a PCR | 3 uL | Kan | N/A | 2 yellow/pink colonies (4mm), many white, 1mm colonies (=neg ctrl) |
| P92b PCR | 3 uL | Kan | N/A | many white, 1mm colonies (= neg ctrl) |
| P93a PCR | 3 uL | Kan | N/A | many white, 1mm colonies (= neg ctrl) |
| P93b PCR | 3 uL | Kan | N/A | 2 yellow/pink colonies (4mm), many white, 1mm colonies (=neg ctrl) |
| P58a A (from E1) | 2 uL | Kan | N/A | many white, 1mm colonies (> neg ctrl) |
| P84a B (from E1) | 3 uL | Kan | N/A | many white, 1mm colonies (> neg ctrl) |
| P28 (TetR, GFP, ColE1) | 2 uL | Kan | N/A | 12 yellow/pink colonies (4mm), many white, 1mm colonies (=neg ctrl) |
| P33 (TetR, Venus, p15a) | 5 uL | Chlor | N/A | blank |
| (+) ctrl, p59B | 1 uL | Kan | N/A | many white, 1mm colonies (TMTC) |
| (-) ctrl, EB buffer | 1 uL | Kan | N/A | many white, 1mm colonies (TMTC) |
Test of IPTG Inducible System
7/15/08 S1 containing 2 vectors one coding for LacI and one w/ a lac promoter controlling GFP expresssion were tested with IPTG
| Strain | OD @ 0h | Fluor @ 0h | Fluor/OD | OD @ 2h no IPTG | Fluor @ 2h no IPTG | Fluor/OD2 | OD @ 2h w/ IPTG | Fluor @ 2h w/ IPTG | Fluor/OD3 | Difference in Levels of Fluor due to IPTG treatment (Fluor/OD3-Fluor/OD3) |
| p59 cells w/ p12 vector A | 0.420 | 572.660 | 1363.476 | 1.150 | 863.790 | 751.122 | 0.818 | 1099.980 | 1344.719 | 593.597 |
| p59 cells w/ p12 vector B | 0.427 | 476.300 | 1115.457 | 1.011 | 905.500 | 895.648 | 1.012 | 1048.150 | 1035.721 | 140.073 |
| p59 cells w/ p13 vector A | 0.474 | 84.440 | 178.143 | 1.047 | 177.100 | 169.150 | 1.026 | 151.450 | 147.612 | -21.538 |
| p59 cells w/ p13 vector B | 0.330 | 79.010 | 239.424 | 1.027 | 171.320 | 166.816 | 0.930 | 122.870 | 132.118 | -34.698 |
| p27 cells w/ p59b vector 1a | 0.364 | 76.050 | 208.929 | 0.615 | 180.420 | 293.366 | 0.775 | 105.070 | 135.574 | -157.792 |
| p27 cells w/ p59b vector 1b | 0.299 | 73.740 | 246.622 | 0.841 | 189.910 | 225.815 | 0.824 | 157.240 | 190.825 | -34.989 |
| p27 cells w/ p59b vector 2a | 0.302 | 67.830 | 224.603 | 0.713 | 170.020 | 238.457 | 0.716 | 105.530 | 147.388 | -91.069 |
| p27 cells w/ p59b vector 2b | 0.476 | 81.830 | 171.912 | 0.683 | 178.370 | 261.157 | 0.647 | 114.140 | 176.414 | -84.742 |
| p13 cells w/ p59b 1a | 0.371 | 126.440 | 340.809 | 0.948 | 186.400 | 196.624 | 0.852 | 111.840 | 131.268 | -65.357 |
| p13 cells w/ p59b 1b | 0.535 | 91.750 | 171.495 | 0.959 | 167.660 | 174.828 | 0.941 | 130.750 | 138.948 | -35.880 |
| p13 cells w/ p59b 1c | 0.431 | 111.460 | 258.608 | 1.016 | 184.350 | 181.447 | 0.937 | 153.330 | 163.639 | -17.808 |
| p13 cells w/ p59b 2a | 0.582 | 73.400 | 126.117 | 0.938 | 183.240 | 195.352 | 0.924 | 124.960 | 135.238 | -60.114 |
| p13 cells w/ p59b 2b | 0.543 | 118.060 | 217.422 | 0.936 | 189.170 | 202.105 | 1.054 | 139.860 | 132.694 | -69.410 |
| p13 cells w/ p59b 2c | 0.523 | 111.600 | 213.384 | 0.992 | 192.720 | 194.274 | 1.160 | 169.380 | 146.017 | -48.257 |
| p34 | 0.045 | 191.140 | 4247.556 | 0.825 | 413.930 | 501.733 | 1.006 | 384.310 | 382.018 | -119.715 |
| p59b (glycerol) + cntl | 1.458 | 131.240 | 90.014 | #DIV/0! | #DIV/0! | #DIV/0! | ||||
| p59b (plate) + cntl | 0.000 | 288.750 | #DIV/0! | 1.025 | 308.480 | 300.956 | 0.996 | 261.480 | 262.530 | -38.426 |
| mtr A negative control | 0.097 | 129.620 | 1336.289 | #DIV/0! | #DIV/0! | #DIV/0! | ||||
| mtrB negative control | 0.000 | 109.910 | #DIV/0! | #DIV/0! | #DIV/0! | #DIV/0! | ||||
| LB | 106.800 | #DIV/0! | 0.000 | 101.160 | #DIV/0! | #DIV/0! | #DIV/0! |
7/17/08:
| Strain | OD @ 0h | Fluor @ 0h | Fluor/OD | OD @ 2h no IPTG | Fluor @ 2h no IPTG | Fluor/OD2 | OD @ 2h w/ IPTG | Fluor @ 2h w/ IPTG | Fluor/OD3 | Difference in Levels of Fluor due to IPTG treatment (Fluor/OD3-Fluor/OD2) |
| LB | 0.000 | 120.050 | #DIV/0! | 0.000 | 110.640 | #DIV/0! | #DIV/0! | #DIV/0! | ||
| WT S1 | 0.232 | 131.340 | 566.121 | 1.003 | 136.600 | 136.191 | 0.700 | 122.420 | 174.886 | 38.694 |
| p59b | 0.038 | 134.080 | 3528.421 | 0.299 | 135.160 | 452.040 | 0.293 | 137.400 | 468.942 | 16.902 |
| p59b w/ p12 | 0.187 | 175.520 | 938.610 | 0.621 | 278.470 | 448.422 | 0.685 | 312.750 | 456.569 | 8.147 |
| p59b w/ p13 | 0.217 | 115.500 | 532.258 | 0.655 | 108.150 | 165.115 | 0.785 | 126.850 | 161.592 | -3.522 |
| p13 w/ p59b 1 | 0.239 | 101.130 | 423.138 | 0.751 | 110.760 | 147.483 | 0.758 | 109.220 | 144.090 | -3.394 |
| p13 w/ p59b 2 | 0.245 | 90.400 | 368.980 | 0.708 | 117.250 | 165.607 | 0.747 | 126.030 | 168.715 | 3.108 |
| p27 w/ p59b 1 | 0.141 | 130.950 | 928.723 | 0.495 | 156.170 | 315.495 | 0.493 | 121.950 | 247.363 | -68.132 |
| p27 w/ p59b 2 | 0.171 | 128.890 | 753.743 | 0.581 | 127.040 | 218.657 | 0.487 | 123.580 | 253.758 | 35.100 |
Overnight cultures 07/18
We made overnight cultures of the parts we got from MIT
- P96
- P95
- P51
- P52
- P17
We also made cultures for plasmids we will need later
- P1 (A and B)
- P3 (A and B)
- P46 (A and B)
- P63 (A and B)
- P48
- P49 (a and B)
- P63 (A and B)
We are not completely sure if the LB Kan we used contains Kan or if it is just plain LB. So we also made a culture of P95, which is Amp resistant, in the this medium. So if it doesn't grow, then Kan was added to that bottle of LB.
07/19: All of these cultures grew except for P46A. The LB Kan really does have Kan.
RE digests and gels 07/18
Gel 1
- Lane 1: 1 kb ladder
- Lane 2: P90α cut SP (~3600)
- Lane 3: P90α uncut
- Lane 4: P90β cut SP (~3600)
- Lane 5: P90β uncut
- Lane 6: P90γ cut SP (~3600)
- Lane 7: P90γ uncut
- Lane 8: P90δ cut SP (~3600)
- Lane 9: P90δ uncut
- Lane 10: ligated P76 cut EX (3857)
- Lane 11: ligated P76 uncut (3857)
Gel 2
- Lane 1: 1 kb ladder
- Lane 2: P90ε cut SP (~3600)
- Lane 3: P90ε uncut
- Lane 4: P90ζ cut SP (~3600)
- Lane 5: P90ζ uncut
- Lane 6: Christina's plasmid uncut (?)
- Lane 7: Christina's plasmid cut EX (?)
- Lane 8: Christina's plasmid cut ES (~1300)
- Lane 9: Christina's plasmid cut XP (~1300)
- Lane 10: ligated P91 cut EX (4327)
- Lane 11: ligated P91 uncut (4327)
- Lane 12: mtrB PCR (2200)
Housekeeping!
Daily
- Pour LB-Amp plates.
- Ensure we have petri dishes, LB, LB agar, and agarose.
- Make LB-antibiotic medias as necessary.
Testing and Making Competent E. coli
Test Transformation of New Batches of Competent Cells 7/15
| Plate | Marker | # Colonies |
| E1 (old) + pUC19 (1 uL) | Amp | >100 |
| E1 (new) + pUC19 (1 uL) | Amp | 10 |
| E2/ TOP10 (new) + pUC19 (1 uL) | Amp | 13 |
| Amp (-) ctrl | Amp | 0 |
Making New Competent Cells 7/22
As per Jason's lab's protocol.
Keeping Up Stocks of Plasmids
7/15/08 Minipreps
| Plasmid/ Sample | ng/uL | A260 | 260/280 | 260/230 | Constant |
| S1 P58b A? | 67.45 | 1.349 | 1.99 | 2.13 | 50 |
| E1 P39A 08 2 | 362.89 | 7.258 | 1.94 | 2.26 | 50 |
| E1 P39A 08 1 | 417.46 | 8.349 | 1.96 | 2.26 | 50 |
| E1 P38a 07 2 | 351.01 | 7.02 | 1.97 | 2.07 | 50 |
| E1 P38a 07 1 | 293.1 | 5.862 | 1.96 | 2.15 | 50 |
| E1 P76 (17+45) | 534.13 | 10.683 | 1.9 | 2.27 | 50 |
| S1 P34 B amp | 941.83 | 18.837 | 1.9 | 2.26 | 50 |
| S1 P34 B amp | 994.7 | 19.894 | 1.91 | 2.26 | 50 |
7/18/08
| Plasmid/ Sample | ng/uL | A260 | 260/280 | 260/230 | Constant |
| S1 P59b 1 | 450.61 | 9.012 | 1.9 | 2.33 | 50 |
| S1 P59b 2 | 328.89 | 6.578 | 1.92 | 2.33 | 50 |
| E1 P58a 1 | 144.21 | 2.884 | 2 | 2.38 | 50 |
| E1 P58a 2 | 91.06 | 1.821 | 1.9 | 2.38 | 50 |
| E1 P80 | 128.63 | 2.573 | 2.04 | 2.26 | 50 |
RE digests 07/19
We digested
- P17, P51, P52 (QPIs) with EX so we can add high/low constitutive promoters
- P63 (terminator) with EX
- P48, P49a, P49B (Amp, Cm resistance cassettes) with XP so we can add them to P90 (P1 + CDF)
| P17 | P51 | P52 | P63A | P63B | P48 | P49A | P49B | |
| DNA | 20 μL | 20 μL | 20 μL | 10 μL | 10 μL | 4 μL | 20 μL | 4 μL |
| 10X NEB Buffer | buffer 2, 2.5 μL | buffer 2, 2.5 μL | buffer 2, 2.5 μL | buffer 2, 2.5 μL | buffer 2, 2.5 μL | buffer 3, 2.5 μL | buffer 3, 2.5 μL | buffer 3, 2.5 μL |
| 25X BSA | 1 μL | 1 μL | 1 μL | 1 μL | 1 μL | 1 μL | 1 μL | 1 μL |
| REs (1 μL each) | EX | EX | EX | EX | EX | XP | XP | XP |
| Water | 0 μL | 0 μL | 0 μL | 9.5 μL | 9.5 μL | 15.5 μL | 0 μL | 15.5 μL |
Transformation of P86, P87, P97
We transformed each of the UTAustin plasmids (individually) and a new strong RBS into TOP 10 cells. The RBS has been miniprepped. The UTA plasmid transformants formed a lawn, so were restreaked first. Liquid cultures have been grown up. P87 requires mutagenesis to remove a PstI site ~100 bp into the coding region of the Cph/EnvZ fusion.








