IGEM:IMPERIAL/2009/Encapsulation/Phase2/Colanic acid: Difference between revisions
(→B3023:) |
James Field (talk | contribs) |
||
| (8 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
==Mucoid Encapsulation== | ==Mucoid Encapsulation== | ||
[[Image:CA.JPG]] | |||
<cite>Colanic11</cite> | |||
==Background== | ==Background== | ||
| Line 110: | Line 114: | ||
*'''[[User:James Chappell|James Chappell]] 10:03, 23 July 2009 (EDT)''':I like this idea, but I feel that your trying to do the job that could automatically done by a timer.As a group I feel you need to review what options there are there for timers...I have not really seen any progress on this aspect.Also generally I would like you to think of decoupling this contorl/timer module from the biosynthesis module. | *'''[[User:James Chappell|James Chappell]] 10:03, 23 July 2009 (EDT)''':I like this idea, but I feel that your trying to do the job that could automatically done by a timer.As a group I feel you need to review what options there are there for timers...I have not really seen any progress on this aspect.Also generally I would like you to think of decoupling this contorl/timer module from the biosynthesis module. | ||
==[http://www.ncbi.nlm.nih.gov/gene/945805?ordinalpos=1&itool=EntrezSystem2.PEntrez.Gene.Gene_ResultsPanel.Gene_RVDocSum B3023:]== | ==[http://www.ncbi.nlm.nih.gov/gene/945805?ordinalpos=1&itool=EntrezSystem2.PEntrez.Gene.Gene_ResultsPanel.Gene_RVDocSum B3023:]== | ||
| Line 123: | Line 127: | ||
RcsB: | RcsB: | ||
<i>DNA (648 bp)</i> | |||
atgaacaacatgaacgtgattattgcggatgatcatccgattgtgctgtttggcattcgc | |||
aaaagcctggaacagattgaatgggtgaacgtggtgggcgaatttgaagatagcaccgcg | |||
ctgattaacaacctgccgaaactggatgcgcatgtgctgattaccgatctgagcatgccg | |||
ggcgataaatatggcgatggcattaccctgattaaatatattaaacgccattttccgagc | |||
ctgagcattattgtgctgaccatgaacaacaacccggcgattctgagcgcggtgctggat | |||
ctggatattgaaggcattgtgctgaaacagggcgcgccgaccgatctgccgaaagcgctg | |||
gcggcgctgcagaaaggcaaaaaatttaccccggaaagcgtgagccgcctgctggaaaaa | |||
attagcgcgggcggctatggcgataaacgcctgagcccgaaagaaagcgaagtgctgcgc | |||
ctgtttgcggaaggctttctggtgaccgaaattgcgaaaaaactgaaccgcagcattaaa | |||
accattagcagccagaaaaaaagcgcgatgatgaaactgggcgtggaaaacgatattgcg | |||
ctgctgaactatctgagcagcgtgaccctgagcccggcggataaagat | |||
<i>Protein</i> | |||
1 mnnmnviiad dhpivlfgir ksleqiewvn vvgefedsta linnlpklda hvlitdlsmp | 1 mnnmnviiad dhpivlfgir ksleqiewvn vvgefedsta linnlpklda hvlitdlsmp | ||
61 gdkygdgitl ikyikrhfps lsiivltmnn npailsavld ldiegivlkq gaptdlpkal | 61 gdkygdgitl ikyikrhfps lsiivltmnn npailsavld ldiegivlkq gaptdlpkal | ||
| Line 132: | Line 149: | ||
B3023: | B3023: | ||
<i>DNA (480bp)</i> | |||
atgaccaacctgaccctggatgtgaacattattgattttccgagcattccggtggcgatg | |||
ctgccgcatcgctgcagcccggaactgctgaactatagcgtggcgaaatttattatgtgg | |||
cgcaaagaaaccggcctgagcccggtgaaccagagccagacctttggcgtggcgtgggat | |||
gatccggcgaccaccgcgccggaagcgtttcgctttgatatttgcggcagcgtgagcgaa | |||
ccgattccggataaccgctatggcgtgagcaacggcgaactgaccggcggccgctatgcg | |||
gtggcgcgccatgtgggcgaactggatgatattagccataccgtgtggggcattattcgc | |||
cattggctgccggcgagcggcgaaaaaatgcgcaaagcgccgattctgtttcattatacc | |||
aacctggcggaaggcgtgaccgaacagcgcctggaaaccgatgtgtatgtgccgctggcg | |||
<i>Protein</i> | |||
1 mtnltldvni idfpsipvam lphrcspell nysvakfimw rketglspvn qsqtfgvawd | 1 mtnltldvni idfpsipvam lphrcspell nysvakfimw rketglspvn qsqtfgvawd | ||
61 dpattapeaf rfdicgsvse pipdnrygvs ngeltggrya varhvgeldd ishtvwgiir | 61 dpattapeaf rfdicgsvse pipdnrygvs ngeltggrya varhvgeldd ishtvwgiir | ||
| Line 138: | Line 166: | ||
Waal Ligase: | Waal Ligase: | ||
<i>DNA (1257bp)</i> | |||
atgctgaccagctttaaactgcatagcctgaaaccgtataccctgaaaagcagcatgatt | |||
ctggaaattattacctatattctgtgcttttttagcatgattattgcgtttgtggataac | |||
acctttagcattaaaatttataacattaccgcgattgtgtgcctgctgagcctgattctg | |||
cgcggccgccaggaaaactataacattaaaaacctgattctgccgctgagcatttttctg | |||
attggcctgctggatctgatttggtatagcgcgtttaaagtggataacagcccgtttcgc | |||
gcgacctatcatagctatctgaacaccgcgaaaatttttatttttggcagctttattgtg | |||
tttctgaccctgaccagccagctgaaaagcaaaaaagaaagcgtgctgtataccctgtat | |||
agcctgagctttctgattgcgggctatgcgatgtatattaacagcattcatgaaaacgat | |||
cgcattagctttggcgtgggcaccgcgaccggcgcggcgtatagcaccatgctgattggc | |||
attgtgagcggcgtggcgattctgtataccaaaaaaaaccatccgtttctgtttctgctg | |||
aacagctgcgcggtgctgtatgtgctggcgctgacccagacccgcgcgaccctgctgctg | |||
tttccgattatttgcgtggcggcgctgattgcgtattataacaaaagcccgaaaaaattt | |||
accagcagcattgtgctgctgattgcgattctggcgagcattgtgattatttttaacaaa | |||
ccgattcagaaccgctataacgaagcgctgaacgatctgaacagctataccaacgcgaac | |||
agcgtgaccagcctgggcgcgcgcctggcgatgtatgaaattggcctgaacatttttatt | |||
aaaagcccgtttagctttcgcagcgcggaaagccgcgcggaaagcatgaacctgctggtg | |||
gcggaacataaccgcctgcgcggcgcgctggaatttagcaacgtgcatctgcataacgaa | |||
attattgaagcgggcagcctgaaaggcctgatgggcatttttagcaccctgtttctgtat | |||
tttagcctgttttatattgcgtataaaaaacgcgcgctgggcctgctgattctgaccctg | |||
ggcattgtgggcattggcctgagcgatgtgattatttgggcgcgcagcattccgattatt | |||
attattagcgcgattgtgctgctgctggtgattaacaaccgcaacaacaccattaac | |||
<i>Protein</i> | |||
1 mltsfklhsl kpytlkssmi leiityilcf fsmiiafvdn tfsikiynit aivcllslil | |||
61 rgrqenynik nlilplsifl iglldliwys afkvdnspfr atyhsylnta kififgsfiv | |||
121 fltltsqlks kkesvlytly slsfliagya myinsihend risfgvgtat gaaystmlig | |||
181 ivsgvailyt kknhpflfll nscavlyvla ltqtratlll fpiicvaali ayynkspkkf | |||
241 tssivlliai lasiviifnk piqnryneal ndlnsytnan svtslgarla myeiglnifi | |||
301 kspfsfrsae sraesmnllv aehnrlrgal efsnvhlhne iieagslkgl mgifstlfly | |||
361 fslfyiaykk ralglliltl givgiglsdv iiwarsipii iisaivlllv innrnntin | |||
==Useful Papers== | ==Useful Papers== | ||
| Line 151: | Line 212: | ||
<biblio>#Colanic9 pmid=19139876</biblio> | <biblio>#Colanic9 pmid=19139876</biblio> | ||
<biblio>#Colanic10 pmid=17227761</biblio> | <biblio>#Colanic10 pmid=17227761</biblio> | ||
<biblio>#Colanic11 pmid=19139876</biblio> | |||
Latest revision as of 08:12, 4 August 2009
Mucoid Encapsulation
[1]
Background
Colanic acid is an exopolysaccharide produced by many bacteria including E.coli.
It forms part of the cell surface slime and is required for the development of the three-dimensional structure and the depth of biofilms.
Since it is naturally produced by E.coli we can manipulate the pathway as opposed to building in a new one (e.g. alginate biosynthesis).
Colanic acid has been shown to offer production against acidic conditions and dessication, it is also non-pathogenic.
- James Chappell 09:51, 23 July 2009 (EDT):Any evidence about how bacteria with this coat would survive through the GI track? Be great if there were some examples, obviously E.coli can survive to some extend as of course we can become infected by some strains but there maybe some more direct evidence out there.
James Field Response:
The following article shows that knocking out colanic acid synthesis decreases viability on acidic media and investigates exposure to bile salts. The paper also makes reference to the fact that the colanic acid capsule confers a strong negative charge which is thought to prevent the migration of protons into the cell. [2]
--SxE00 05:49, 24 July 2009 (EDT)
I have a big concern here: isn't the purpose of the protection offered by the biofilm to keep enough of the cells alive so the colony may survive the temporariliy harsh conditions?
But our cells will be dead, hence won't mutiply - the article does not seem to correct for cell growth, but I may be wrong.
Worse still we may have destroyed the walls so the enzyme we will have manufactured will be only protected by the biofilm.
Overall, the efficiency of our encapsulation will be far worse than the figures in the article.
Please tell me I have missed something!
Matthieu (I know, the login is bizarre)
Structure
It is a polyanionic heteropolysaccharide containing a repeat unit with D-glucose, L-fucose, D-galactose, and D-glucuronate sugars that are nonstoichiometrically decorated with O-acetyl and pyruvate side chains.
Structure after carboxyl esterification:
Acid resistance
Colanic acid is shown to increase acid resistance of bacteria. Indeed, colanic acid biosynthesis is up-regulated during the storage of bacteria in acidic foods such as yogurt.[3]
Comparing between mutant E.coli O157:H7 and E.coli O157:H7 that produce colanic acid.
CA confers a strong negative charge to the cell surface which, we suggest may serve as a buffer by neutralizing protons at the cell surface, whereby preventing positively charged chemical groups from accumulating on cell envelopes and from penetrating into cells. We further hypothesize that the amount of CA on cell surfaces determines the buffering capacity of cells. When cells lose their ability to produce CA, cell surfaces become less negatively charged and thereby have reduced buffering capacity. When negatively charged cell surfaces are neutralized, protons will accumulate and enter cells freely. Such a change in intracellular pH will impair cell metabolism, causing cell death. [4]
- --SxE00 09:16, 24 July 2009 (EDT)
your cells will be dead...
Membrane Tethering
Unlike other exopolysaccharides, colanic acid does not naturally bind to the cell surface but rather forms a thick mesh between cells. We must adjust colanic acid production to ensure that cells are encapsulated. It has recently been shown that E.coli K12 can link colanic acid polymers to the lipid A core.[5]
Interestingly, pathogenic strains of E.coli usually link their O-antigens to the lipid A core molecule but becuase the O-antigen is absent from E.coli K-12 (due to an IS5 insertion) it is possible to substitute in the colanic acid. This membrane tethered colanic acid is called Mlps.
O-Antigen:
Membrane Tethered Colanic Acid (Mlps):
Regulation
It should be noted that colanic acid production is upregulated in response to low temperatures, osmotic shock, dessication and blue light.[6]
Colanic acid is not produced under optimum conditions or inside a living host.
Colanic acid biosynthesis is facilitated by the wca (cps) gene cluster that contains 19 genes and is regulated by rcs phospho-relay system.
RcsB:
Upregulation of RcsB has been shown to induce colanic acid production.[7] Colanic acid production is also upregulated by blue light.[8]
We can decouple this pathway to control the thickness of our cellular capsule.
To efficiently link an input of blue light to an output of colanic acid, the circuit can be simplified by placing RcsB under the control of YcgE repressible promoter. This biobrick has been designed by the 2009 Leuven team (BBa_K238000). There is currently no BioBrick for RcsB although the sequence is known.
There are also RcsB knockout strains of E.coli which could be used to avoid cross-talk between pathways.
http://cgsc2.biology.yale.edu/Mutation.php?ID=77155
There was also a recent 2009 paper that used a RcsB knockout strain of E.coli. [9]
- James Chappell 10:01, 23 July 2009 (EDT):Cool but the paper you have cited above shows that by contorlling the RscB you will effect your cell division so defiantly we need to consider this, is it okay to write quick summary of the paper and what unintended affects we might expect. Also it seemed from the diagram that RscB is really at a node to many different pathways, look for recent paper to see if they have filled out any of the downstream pathways more.
James Field Response: RcsB is indeed nodal and while this brings with it important considerations (e.g. cell division), it facilitates the activation of the ugd operon which is required for capsule synthesis. It also upregulates a number of acid tolerance proteins that might be advantageous. It is strongly agreed that more research needs to be done with respect to the global effect of RcsB.
It might also be interesting to put an amber stop codon in the RcsB gene and co-express SupD with our gene of interest. This way, capsule formation would be influenced by both a light input and the levels of the gene of interest. Having said this, you only have to look at mucoid colonies to see the excessive colanic acid production, this indicates that all cells within a high density population will become encapsulated (i.e. it will be difficult to efficiently relate the presence of a capsule to a discrete amount of protein).
Based on this, it might be better to add an inducer which initiates the production of a protein of interest at a known rate. After a certain period of time, a blue light is switched on which does two things:
1) Initiates encapsulation (& possibly trehalose synthesis).
2) Turns off the production of the protein of interest. This could be achieved by using blue light to trigger the expression of a repressor which binds to the promoter controlling the protein of interest. Of course this level of dosage control is dependent on our final application. For instance, if the xylanase/cellulase application were chosen, higher levels of protein would be advantageous and therefore it would not be necessary to repress synthesis upon encapsulation.
- James Chappell 10:03, 23 July 2009 (EDT):I like this idea, but I feel that your trying to do the job that could automatically done by a timer.As a group I feel you need to review what options there are there for timers...I have not really seen any progress on this aspect.Also generally I would like you to think of decoupling this contorl/timer module from the biosynthesis module.
B3023:
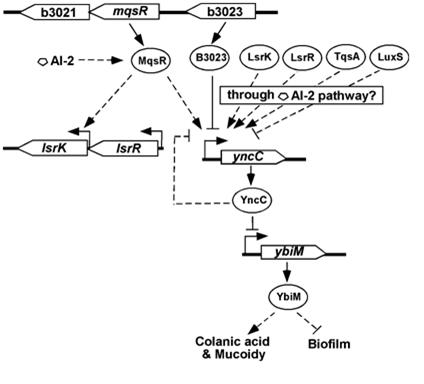
A recent Nature paper has also shown that colanic acid capsule production can also be greatly upregulated via the expression of a putative transcription factor (B3023). [10]
Sequences
RcsB:
DNA (648 bp)
atgaacaacatgaacgtgattattgcggatgatcatccgattgtgctgtttggcattcgc
aaaagcctggaacagattgaatgggtgaacgtggtgggcgaatttgaagatagcaccgcg
ctgattaacaacctgccgaaactggatgcgcatgtgctgattaccgatctgagcatgccg
ggcgataaatatggcgatggcattaccctgattaaatatattaaacgccattttccgagc
ctgagcattattgtgctgaccatgaacaacaacccggcgattctgagcgcggtgctggat
ctggatattgaaggcattgtgctgaaacagggcgcgccgaccgatctgccgaaagcgctg
gcggcgctgcagaaaggcaaaaaatttaccccggaaagcgtgagccgcctgctggaaaaa
attagcgcgggcggctatggcgataaacgcctgagcccgaaagaaagcgaagtgctgcgc
ctgtttgcggaaggctttctggtgaccgaaattgcgaaaaaactgaaccgcagcattaaa
accattagcagccagaaaaaaagcgcgatgatgaaactgggcgtggaaaacgatattgcg
ctgctgaactatctgagcagcgtgaccctgagcccggcggataaagat
Protein
1 mnnmnviiad dhpivlfgir ksleqiewvn vvgefedsta linnlpklda hvlitdlsmp
61 gdkygdgitl ikyikrhfps lsiivltmnn npailsavld ldiegivlkq gaptdlpkal
121 aalqkgkkft pesvsrllek isaggygdkr lspkesevlr lfaegflvte iakklnrsik
181 tissqkksam mklgvendia llnylssvtl spadkd
B3023:
DNA (480bp)
atgaccaacctgaccctggatgtgaacattattgattttccgagcattccggtggcgatg
ctgccgcatcgctgcagcccggaactgctgaactatagcgtggcgaaatttattatgtgg
cgcaaagaaaccggcctgagcccggtgaaccagagccagacctttggcgtggcgtgggat
gatccggcgaccaccgcgccggaagcgtttcgctttgatatttgcggcagcgtgagcgaa
ccgattccggataaccgctatggcgtgagcaacggcgaactgaccggcggccgctatgcg
gtggcgcgccatgtgggcgaactggatgatattagccataccgtgtggggcattattcgc
cattggctgccggcgagcggcgaaaaaatgcgcaaagcgccgattctgtttcattatacc
aacctggcggaaggcgtgaccgaacagcgcctggaaaccgatgtgtatgtgccgctggcg
Protein
1 mtnltldvni idfpsipvam lphrcspell nysvakfimw rketglspvn qsqtfgvawd
61 dpattapeaf rfdicgsvse pipdnrygvs ngeltggrya varhvgeldd ishtvwgiir
121 hwlpasgekm rkapilfhyt nlaegvteqr letdvyvpla
Waal Ligase:
DNA (1257bp)
atgctgaccagctttaaactgcatagcctgaaaccgtataccctgaaaagcagcatgatt
ctggaaattattacctatattctgtgcttttttagcatgattattgcgtttgtggataac
acctttagcattaaaatttataacattaccgcgattgtgtgcctgctgagcctgattctg
cgcggccgccaggaaaactataacattaaaaacctgattctgccgctgagcatttttctg
attggcctgctggatctgatttggtatagcgcgtttaaagtggataacagcccgtttcgc
gcgacctatcatagctatctgaacaccgcgaaaatttttatttttggcagctttattgtg
tttctgaccctgaccagccagctgaaaagcaaaaaagaaagcgtgctgtataccctgtat
agcctgagctttctgattgcgggctatgcgatgtatattaacagcattcatgaaaacgat
cgcattagctttggcgtgggcaccgcgaccggcgcggcgtatagcaccatgctgattggc
attgtgagcggcgtggcgattctgtataccaaaaaaaaccatccgtttctgtttctgctg
aacagctgcgcggtgctgtatgtgctggcgctgacccagacccgcgcgaccctgctgctg
tttccgattatttgcgtggcggcgctgattgcgtattataacaaaagcccgaaaaaattt
accagcagcattgtgctgctgattgcgattctggcgagcattgtgattatttttaacaaa
ccgattcagaaccgctataacgaagcgctgaacgatctgaacagctataccaacgcgaac
agcgtgaccagcctgggcgcgcgcctggcgatgtatgaaattggcctgaacatttttatt
aaaagcccgtttagctttcgcagcgcggaaagccgcgcggaaagcatgaacctgctggtg
gcggaacataaccgcctgcgcggcgcgctggaatttagcaacgtgcatctgcataacgaa
attattgaagcgggcagcctgaaaggcctgatgggcatttttagcaccctgtttctgtat
tttagcctgttttatattgcgtataaaaaacgcgcgctgggcctgctgattctgaccctg
ggcattgtgggcattggcctgagcgatgtgattatttgggcgcgcagcattccgattatt
attattagcgcgattgtgctgctgctggtgattaacaaccgcaacaacaccattaac
Protein
1 mltsfklhsl kpytlkssmi leiityilcf fsmiiafvdn tfsikiynit aivcllslil
61 rgrqenynik nlilplsifl iglldliwys afkvdnspfr atyhsylnta kififgsfiv
121 fltltsqlks kkesvlytly slsfliagya myinsihend risfgvgtat gaaystmlig
181 ivsgvailyt kknhpflfll nscavlyvla ltqtratlll fpiicvaali ayynkspkkf
241 tssivlliai lasiviifnk piqnryneal ndlnsytnan svtslgarla myeiglnifi
301 kspfsfrsae sraesmnllv aehnrlrgal efsnvhlhne iieagslkgl mgifstlfly
361 fslfyiaykk ralglliltl givgiglsdv iiwarsipii iisaivlllv innrnntin
Useful Papers
- Gervais FG, Phoenix P, and Drapeau GR. The rcsB gene, a positive regulator of colanic acid biosynthesis in Escherichia coli, is also an activator of ftsZ expression. J Bacteriol. 1992 Jun;174(12):3964-71. DOI:10.1128/jb.174.12.3964-3971.1992 |
- Mao Y, Doyle MP, and Chen J. Role of colanic acid exopolysaccharide in the survival of enterohaemorrhagic Escherichia coli O157:H7 in simulated gastrointestinal fluids. Lett Appl Microbiol. 2006 Jun;42(6):642-7. DOI:10.1111/j.1472-765X.2006.01875.x |
- Tschowri N, Busse S, and Hengge R. The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes Dev. 2009 Feb 15;23(4):522-34. DOI:10.1101/gad.499409 |
- Callewaert L, Vanoirbeek KG, Lurquin I, Michiels CW, and Aertsen A. The Rcs two-component system regulates expression of lysozyme inhibitors and is induced by exposure to lysozyme. J Bacteriol. 2009 Mar;191(6):1979-81. DOI:10.1128/JB.01549-08 |
- Mao Y, Doyle MP, and Chen J. Insertion mutagenesis of wca reduces acid and heat tolerance of enterohemorrhagic Escherichia coli O157:H7. J Bacteriol. 2001 Jun;183(12):3811-5. DOI:10.1128/JB.183.12.3811-3815.2001 |
- Mao Y, Doyle MP, and Chen J. Role of colanic acid exopolysaccharide in the survival of enterohaemorrhagic Escherichia coli O157:H7 in simulated gastrointestinal fluids. Lett Appl Microbiol. 2006 Jun;42(6):642-7. DOI:10.1111/j.1472-765X.2006.01875.x |
- Lee SM and Chen J. Survival of Escherichia coli O157:H7 in set yogurt as influenced by the production of an exopolysaccharide, colanic acid. J Food Prot. 2004 Feb;67(2):252-5. DOI:10.4315/0362-028x-67.2.252 |
- Zhang XS, García-Contreras R, and Wood TK. Escherichia coli transcription factor YncC (McbR) regulates colanic acid and biofilm formation by repressing expression of periplasmic protein YbiM (McbA). ISME J. 2008 Jun;2(6):615-31. DOI:10.1038/ismej.2008.24 |
- Navasa N, Rodríguez-Aparicio L, Martínez-Blanco H, Arcos M, and Ferrero MA. Temperature has reciprocal effects on colanic acid and polysialic acid biosynthesis in E. coli K92. Appl Microbiol Biotechnol. 2009 Mar;82(4):721-9. DOI:10.1007/s00253-008-1840-4 |