Lerou:Research: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
(New page: {{LerouLab}} {|cellspacing="5" cellpadding="10" style="background:#000033; width: 750px;" |-valign="top" |style="background:#ffffff"| ==Research Goals== Our research goals are to advance...) |
No edit summary |
||
| Line 3: | Line 3: | ||
|-valign="top" | |-valign="top" | ||
|style="background:#ffffff"| | |style="background:#ffffff"| | ||
[[Image:lerou_image_3.png|thumb|right|Embryonic Stem Cell Colony]] | |||
==Research Goals== | ==Research Goals== | ||
| Line 16: | Line 16: | ||
==Outcomes/Applications== | ==Outcomes/Applications== | ||
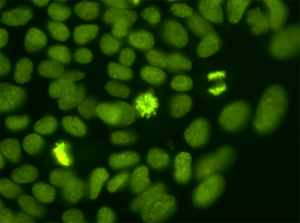
[[Image:lerou_image_2.png|thumb|left|Embryonic Stem Cells, DNA labeled with GFP]] | |||
The most common and clinically problematic form of genomic instability is aneuploidy, a hallmark of cancer and a cause of infertility and congenital developmental defects. The causes of aneuploidy remain largely unknown, yet the impact on development is profound. Aneuploidy affects 35% of clinically recognized spontaneous abortions, 4% of stillborns and 3 in 1000 live borns. As neonatologists, we witness firsthand the consequences of aneuploidy on development when we care for couples that have struggled with infertility and whose infants are afflicted by genetic syndromes. The long term goal of our research will be to use human pluripotent stem cell research to improve our understanding of early human development and how genomic instability disrupts normal development and contributes to birth defects. | The most common and clinically problematic form of genomic instability is aneuploidy, a hallmark of cancer and a cause of infertility and congenital developmental defects. The causes of aneuploidy remain largely unknown, yet the impact on development is profound. Aneuploidy affects 35% of clinically recognized spontaneous abortions, 4% of stillborns and 3 in 1000 live borns. As neonatologists, we witness firsthand the consequences of aneuploidy on development when we care for couples that have struggled with infertility and whose infants are afflicted by genetic syndromes. The long term goal of our research will be to use human pluripotent stem cell research to improve our understanding of early human development and how genomic instability disrupts normal development and contributes to birth defects. | ||
Revision as of 05:12, 9 March 2011