Lidstrom:Transformation: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
No edit summary |
|||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
Back to [[Lidstrom:Protocols|Protocols]] | Back to [[Lidstrom:Protocols|Protocols]] | ||
== Electroporation is much more efficient than chemical transformation == | |||
* Transformations done with 1uL of 0.1 ng/uL DNA (pGA 3K3 RFP from Rob Egbert). 0.1 ng is ~10^8 plasmid copies. Tami's electrocomp cells yielded several hundred colonies, which suggests the transformation efficiency is on the order of 100/10^8 = 0.000001 = 0.0001% efficiency. The chemically competent cells yielded < 10 colonies, which is much lower efficiency. | |||
* [[image:2013_10_14_comparison_of_electroporation_and_chemical_transformation.jpg|thumb|center|upright=2.0|Top10 cells prepared for electroporation and chemical transformation. Both were transformed with ~10^8 copies of DNA (0.1 ng of plasmid.)]] | |||
==Using Chemically Competent Cells== | ==Using Chemically Competent Cells== | ||
| Line 24: | Line 27: | ||
==== Best choice: buy autoclavable ones ==== | ==== Best choice: buy autoclavable ones ==== | ||
* We just bought [http://www.coleparmer.com/Product/Sterile_Electroporation_Cuvette_1_mm_gap_width_white_cap_50_pack/EW-25714-00 these] (7/24/2012) | * We just bought [http://www.coleparmer.com/Product/Sterile_Electroporation_Cuvette_1_mm_gap_width_white_cap_50_pack/EW-25714-00 these] (7/24/2012) but are likely to switch to [http://www.bulldog-bio.com/cuvettes.html these]. | ||
==== Sterilizing | ==== Sterilizing cuvettes for reuse ==== | ||
The cuvettes should be washed for reuse. If washed properly, they can be reused up to 10 times. For each cuvette, do the following while waiting for the cells to recover: | |||
# Squirt 95% ethanol to fill cuvette more than halfway. Add cap and invert cuvette a few times to disinfect it. Pour out in waste receptacle. | |||
# Wash cuvette three times with DI H2O using squeeze bottle and dump water into sink. | |||
# Squirt 95% ethanol in the cuvette, add the cap, and let it sit for an hour or overnight. | |||
# Pour out ethanol and place in UV hood to dry. | |||
# Store in freezer for next use | |||
It seems that the cuvettes rust when left to soak in solution, increasing their rate of arcing. | |||
''Note:'' most cuvettes are made from plastic that will melt. Do not autoclave. | |||
===Transforming Cells=== | ===Transforming Cells=== | ||
| Line 49: | Line 56: | ||
** Transfer cells to sterile 1.5 mL tubes filled with [[SOC| SOC media]] that have been chilled on ice | ** Transfer cells to sterile 1.5 mL tubes filled with [[SOC| SOC media]] that have been chilled on ice | ||
** Record time constant displayed by machine (should be ~ 5-6) | ** Record time constant displayed by machine (should be ~ 5-6) | ||
*** Alternately, add ~950 uL ice cold [[SOC| SOC media]] to cuvette & transfer to ice-cold sterilized cuvette as fast of possible. | *** Alternately, add ~950 uL ice cold [[SOC| SOC media]] (LB is ok) to cuvette & transfer to ice-cold sterilized cuvette as fast of possible. | ||
***Note: Janet is going to try just using LB instead to save prep time. Mila/Marina guesstimated that using SOC may give you ~2 fold better yield than SOC. | ***Note: Janet is going to try just using LB instead to save prep time. Mila/Marina guesstimated that using SOC may give you ~2 fold better yield than SOC. | ||
***Fast --> less cells die. | ***Fast --> less cells die. | ||
| Line 58: | Line 65: | ||
**Leave remaining SOC-cell mixture on the benchtop overnight. | **Leave remaining SOC-cell mixture on the benchtop overnight. | ||
**If you don't have any transformants, plate the rest of the transformation in the morning. | **If you don't have any transformants, plate the rest of the transformation in the morning. | ||
*Note: you will usually get plenty of colonies by using TB (not SOC media, not chilling cuvettes, not icing sample after applying voltage, not pre-warming plates. | |||
====Notes==== | ====Notes==== | ||
| Line 71: | Line 79: | ||
== Transforming Multiple Plasmids == | == Transforming Multiple Plasmids == | ||
* In theory you can transform 2-3 plasmids at once. In practice, it can be difficult. | * In theory you can transform 2-3 plasmids at once. In practice, it can be difficult. | ||
** If Each transformation is 10% efficient, then you would have about (0.10^3)*100% = 0.1% efficiency. [[User: Janet B. Matsen|Janet]] | ** If Each transformation is 10% efficient, then you would have about (0.10^3)*100% = 0.1% efficiency. [[User: Janet B. Matsen|Janet]] tried transforming 3 a few times (7/2012) and never got a single colony. But do try and tell me if you succeed! | ||
* Use electrocompetent cells because they tend to have higher efficiency. | * Use electrocompetent cells because they tend to have higher efficiency. | ||
* Do some cuvettes with just one plasmid as a control & backup. | * Do some cuvettes with just one plasmid as a control & backup. | ||
Latest revision as of 18:14, 14 October 2013
Back to Protocols
Electroporation is much more efficient than chemical transformation
- Transformations done with 1uL of 0.1 ng/uL DNA (pGA 3K3 RFP from Rob Egbert). 0.1 ng is ~10^8 plasmid copies. Tami's electrocomp cells yielded several hundred colonies, which suggests the transformation efficiency is on the order of 100/10^8 = 0.000001 = 0.0001% efficiency. The chemically competent cells yielded < 10 colonies, which is much lower efficiency.

Top10 cells prepared for electroporation and chemical transformation. Both were transformed with ~10^8 copies of DNA (0.1 ng of plasmid.)
Using Chemically Competent Cells
- Thaw frozen (-80oC) competent cells on ice.
- You REALLY dont want it to get too warm; add plasmids while it is still slushy.
- Add 1-10 uL DNA
- 10 uL if ligated plasmid. Use only 1 uL if regular plasmid from miniprep
- Incubate @ 42oC for 45 sec - 1 min
- Incubate on ice for 2 min
- Add 1 mL LB
- Sandy, Ceci, & Janet use 500 uL
- Incubate at 37oC for 45 min - 1 hr in eppendorf tubes
- Ideally shaking though it may not matter. You can tape your tubes to a rack in the shaker. Tape them well if you do -- they fly off!
- Pellet cells by centrifugation
- keep the ~100 uL droplet after you pour it off (Andrew)
- Plate 50-100 uL cells on LB (+antibiotic(s)) agar plate
- If you are worried about having a lawn, do one plate with more cells and dilute a fraction of the cells and plate a diluted aliquot.
Electrocompetent Cells (in progress)
Preparing Cuvettes
Best choice: buy autoclavable ones
Sterilizing cuvettes for reuse
The cuvettes should be washed for reuse. If washed properly, they can be reused up to 10 times. For each cuvette, do the following while waiting for the cells to recover:
- Squirt 95% ethanol to fill cuvette more than halfway. Add cap and invert cuvette a few times to disinfect it. Pour out in waste receptacle.
- Wash cuvette three times with DI H2O using squeeze bottle and dump water into sink.
- Squirt 95% ethanol in the cuvette, add the cap, and let it sit for an hour or overnight.
- Pour out ethanol and place in UV hood to dry.
- Store in freezer for next use
It seems that the cuvettes rust when left to soak in solution, increasing their rate of arcing. Note: most cuvettes are made from plastic that will melt. Do not autoclave.
Transforming Cells
- use ice cold electrocompetent cells
- mix in 1-2 uL DNA; gently mix to prevent shear stress on cells & DNA
- transfer mixture to sterile cuvette
- make sure there are no bubbles or it could arc (bad for your cells)
- settings for E. Coli:
- brown-caped 1 mm gap tubes:
- 1.8 kV, 200 Ohms, 25 uF.
- green-capped 2 mm cuvette: (in case you don't have brown cuvettes ready)
- 2.5 to 3.0 KV, 200 Ohms, 25 uF
- brown-caped 1 mm gap tubes:
- Shock them by pressing both buttons on our BioRad electroporation machine.
- Press long enough to hear the machine beep.
- It flashes "Ch 9" and has a sustained beep until you release the orange buttons.
- Transfer cells to sterile 1.5 mL tubes filled with SOC media that have been chilled on ice
- Record time constant displayed by machine (should be ~ 5-6)
- Alternately, add ~950 uL ice cold SOC media (LB is ok) to cuvette & transfer to ice-cold sterilized cuvette as fast of possible.
- Note: Janet is going to try just using LB instead to save prep time. Mila/Marina guesstimated that using SOC may give you ~2 fold better yield than SOC.
- Fast --> less cells die.
- Press long enough to hear the machine beep.
- Chill sample on ice for 2 mins to permit the cells to recover.
- Transfer eppendorf tube to 37°C incubator and shake to promote aeration. Incubate for 1 hr to permit expression of antibiotic resistance gene.
- Plate transformation onto prewarmed LB-agar plate supplemented with appropriate antibiotic. I generally plate 200μL but appropriate plating volume depends on efficiency of the transformation.
- Incubate plate overnight at 37°C.
- Leave remaining SOC-cell mixture on the benchtop overnight.
- If you don't have any transformants, plate the rest of the transformation in the morning.
- Note: you will usually get plenty of colonies by using TB (not SOC media, not chilling cuvettes, not icing sample after applying voltage, not pre-warming plates.
Notes
- When choosing the # of vials and cuvettes, you can include 2 vials for two negative controls (one with no DNA added, and another with only cut vector added).
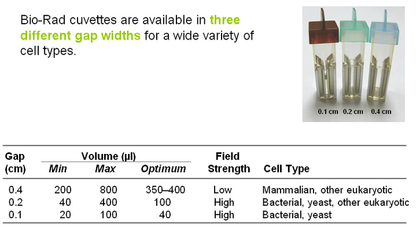
- Cuvette types: (see pic on right)
- BioRad Movie about electroporation. Though it is made for a different machine than we have, the principles are applicable.
- Electroporation is a better strategy for transforming with multiple plasmids. The higher efficiency makes it more likely that some cells will receive all pf the (2+) plasmids.
Transforming Multiple Plasmids
- In theory you can transform 2-3 plasmids at once. In practice, it can be difficult.
- If Each transformation is 10% efficient, then you would have about (0.10^3)*100% = 0.1% efficiency. Janet tried transforming 3 a few times (7/2012) and never got a single colony. But do try and tell me if you succeed!
- Use electrocompetent cells because they tend to have higher efficiency.
- Do some cuvettes with just one plasmid as a control & backup.
- Extra tips:
- Arcing is a problem when you have salts. Wash your competent cells very thoroughly & wash during minipreps very thoroughly.
- Don't add too much miniprepped DNA. Though more DNA --> higher efficiency, more DNA also --> arcing.
- If a sample arcs, plate it anyway. Sometimes you get survivors!
- Have a backup plan
- If you can plan ahead enough to transform them in one at a time, do so. Example: if you are making a 3rd plasmid, but you have two ready, start transforming the first 2 in ASAP. You can try all 3 at once upon completion of the 3rd, but if it fails you will be set back several days.