Lidstrom:Transformation
From OpenWetWare
Back to Protocols
You can chose between chemically competent cells and electrocompetent cells. Andrew makes chemically competent cells for the lab to use. Several strains are kept in stock in the -80oC freezer.
Using Chemically Competent Cells
- Thaw frozen (-80oC) competent cells on ice.
- You REALLY dont want it to get too warm; add plasmids while it is still slushy.
- Add 1-10 uL DNA
- 10 uL if ligated plasmid. Use only 1 uL if regular plasmid from miniprep
- Incubate @ 42oC for 45 sec - 1 min
- Incubate on ice for 2 min
- Add 1 mL LB
- Sandy, Ceci, & Janet use 500 uL
- Incubate at 37oC for 45 min - 1 hr in eppendorf tubes
- Ideally shaking though it may not matter. You can tape your tubes to a rack in the shaker. Tape them well if you do -- they fly off!
- Pellet cells by centrifugation
- keep the ~100 uL droplet after you pour it off (Andrew)
- Plate 50-100 uL cells on LB (+antibiotic(s)) agar plate
- If you are worried about having a lawn, do one plate with more cells and dilute a fraction of the cells and plate a diluted aliquot.
Electrocompetent Cells (in progress)
Preparing Cuvettes
Best choice: buy autoclavable ones
- We just bought these (7/24/2012)
Sterilizing BioRad cuvettes
- Soak in water, rinse with DI water, fill with EtOH overnight, discard EtOH, and dry under UV light.
- Do not autoclave: plastic will melt.
- Use brown capped cuvettes for E. Coli.
- Janet called BioRad (4/2012) and they said ethanol sterilization is fine, but do not autoclave or the plastic will melt. They want you to buy new ones every time instead, but that is silly.
Transforming Cells
- use ice cold electrocompetent cells
- mix in 1-2 uL DNA; gently mix to prevent shear stress on cells & DNA
- transfer mixture to sterile cuvette
- make sure there are no bubbles or it could arc (bad for your cells)
- settings for E. Coli:
- brown-caped 1 mm gap tubes:
- 1.8 kV, 200 Ohms, 25 uF.
- green-capped 2 mm cuvette: (in case you don't have brown cuvettes ready)
- 2.5 to 3.0 KV, 200 Ohms, 25 uF
- brown-caped 1 mm gap tubes:
- Shock them by pressing both buttons on our BioRad electroporation machine.
- Press long enough to hear the machine beep.
- It flashes "Ch 9" and has a sustained beep until you release the orange buttons.
- Transfer cells to sterile 1.5 mL tubes filled with SOC media that have been chilled on ice
- Record time constant displayed by machine (should be ~ 5-6)
- Alternately, add ~950 uL ice cold SOC media to cuvette & transfer to ice-cold sterilized cuvette as fast of possible.
- Note: Janet is going to try just using LB instead to save prep time. Mila/Marina guesstimated that using SOC may give you ~2 fold better yield than SOC.
- Fast --> less cells die.
- Press long enough to hear the machine beep.
- Chill sample on ice for 2 mins to permit the cells to recover.
- Transfer eppendorf tube to 37°C incubator and shake to promote aeration. Incubate for 1 hr to permit expression of antibiotic resistance gene.
- Plate transformation onto prewarmed LB-agar plate supplemented with appropriate antibiotic. I generally plate 200μL but appropriate plating volume depends on efficiency of the transformation.
- Incubate plate overnight at 37°C.
- Leave remaining SOC-cell mixture on the benchtop overnight.
- If you don't have any transformants, plate the rest of the transformation in the morning.
Notes
- When choosing the # of vials and cuvettes, you can include 2 vials for two negative controls (one with no DNA added, and another with only cut vector added).
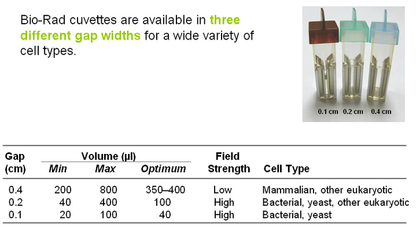
- Cuvette types: (see pic on right)
- BioRad Movie about electroporation. Though it is made for a different machine than we have, the principles are applicable.
- Electroporation is a better strategy for transforming with multiple plasmids. The higher efficiency makes it more likely that some cells will receive all pf the (2+) plasmids.
Transforming Multiple Plasmids
- In theory you can transform 2-3 plasmids at once. In practice, it can be difficult.
- If Each transformation is 10% efficient, then you would have about (0.10^3)*100% = 0.1% efficiency. Janet tried transforming 3 a few times (7/2012) and never got a single colony. But do try and tell me if you succeed!
- Use electrocompetent cells because they tend to have higher efficiency.
- Do some cuvettes with just one plasmid as a control & backup.
- Extra tips:
- Arcing is a problem when you have salts. Wash your competent cells very thoroughly & wash during minipreps very thoroughly.
- Don't add too much miniprepped DNA. Though more DNA --> higher efficiency, more DNA also --> arcing.
- If a sample arcs, plate it anyway. Sometimes you get survivors!
- Have a backup plan
- If you can plan ahead enough to transform them in one at a time, do so. Example: if you are making a 3rd plasmid, but you have two ready, start transforming the first 2 in ASAP. You can try all 3 at once upon completion of the 3rd, but if it fails you will be set back several days.