M465:Bootcamp
Microbiology Boot Camp
In this first lab you will learn:
- Who microbiologists are and what they do
- Aseptic technique: don't contaminate yourself or your cultures!
- The basic equipment and procedures used in microbiological investigation
- Using a lab notebook to record the progress of your experiments
Introduction To Microbiology
Welcome to the unseen world of microorganisms. For most of us, microbes are out of sight and out of mind; largely, the human population would prefer it that way. However, since microbes have a major and continuing impact on us and on our planet, it behooves us to understand them better. By the end of this course, you will. Understanding the microbial world is a huge undertaking. A discipline that defines its scope as including all life forms (and some non-life forms like viruses and prions) that are invisible to the unaided human eye is a bit like saying we will study all humans and other animals shorter than 4 ft. Besides including a huge number of members, the diversity of such a group is overwhelming.
So where do we begin in the study of microbiology? It's good to start with appreciating the power of these tiny, unseen life forms to thrive and spread without our permission or knowledge. It is also wise to recognize that although a tiny fraction of the microbes in our world are disease causing, there are devastating infections caused by microbial pathogens. Although none of the microorganisms that we will knowingly work with this semester are commonly human pathogens, we require that you read and agree to certain rules for working in the microbiology lab that are designed to keep you from infecting yourself, your classmates, and the community. We will also begin today to learn aseptic techniques that will reduce the chance of contaminating your cultures or the chance that your cultures will contaminate you.
Introduction To the Tools and Techniques of Microbiology
Whether you are trying to keep a desired organism from being overgrown by a contaminant, or you are attempting to prevent contaminating yourself, your lab bench, or your lab partner with your cultures; awareness of potential sources of contamination in a microbiology lab is critical. Your success in the lab depends on being open to learning and adopting the standard procedures used in microbiology. Today you will practice aseptic transfer technique and immediately assess your success.
Aseptic transfer
Please watch your instructors demonstrate aseptic transfer. You will be performing this transfer in the hoods in order to minimize contamination. After a demonstration by your instructor, you will practice the basics of aspetic transfer techniques and assess your success.
Broth to Broth or Broth to Plate Transfer
YouTube demo [1]
But keep in mind that in that demo they use a bunsen burner instead of an incinerator and also, we will be performing our inoculations in a laminar flow hood.
Your instructor will demonstrate Broth to Broth and Broth to Plate transfers (protocols found below)
Follow along and pay close attention to how this is done. Contamination in your transfers and your inoculations can cost you the success of your project.
Protocol I: Broth to Broth
1. Label the destination container for the culture (uninoculated sterile broth in a tube or solid medium in a plate).
2. Holding your loop like a pencil, insert the loop into the incinerator. Keep the wire in the flame until it is red-hot. The wire will now be sterile. Allow the loop to cool for a few seconds in the air before touching it to your culture or medium.
3. Pick up the donor broth culture tube with your other hand, while still holding the sterile loop. With the hand holding the loop, use your little finger against your palm to remove the cover or plug from the culture tube as shown in Figure 1. Do not put the cover or plug down on your bench. Now, insert the loop into the broth without touching the sides of the tube, and then remove it, carrying a loopful of culture.

Figure 1: Transferring a culture. (a) Removal of a tube cap while manipulating a loop; (b) Obtaining inoculum from a broth tube while maintaining sterility of the cap (note cap in hand).
4. Pick up the labeled sterile destination tube or plate. Remove its cover.
5. Insert the loop containing the culture into the destination tube of sterile broth, swirl gently and remove. If the destination is a plate of solid medium, follow the directions for streaking for isolation or for a spread plate found below (part II).
6. Replace the cover and set the newly inoculated broth tube in the rack.
7. Re-sterilize the loop before putting it down by inserting the loop into the incinerator. Doing this slowly allows any liquid remaining on the loop to evaporate rather than boil and avoid splattering live bacterial cells all over the bench and you.
Protocol II: Broth to Plate
Nutrient agar plates can be streaked using a three-phase or a four phase pattern (Figure A-1). YouTube Demo [2]. The handling of the plate can be accomplished in a number of ways, all of which attempt to minimize possible contamination by either keeping the lid over the plate or by keeping the plate upside down (Figure A-2).
1. If using a loop, sterilize it first and allow the loop to cool for a few seconds. Not allowing any cap, lid, or plug to leave your hand, dip your loop in the donor broth culture or touch your loop to a colony of bacteria on a streak plate.
2. Using the loop, streak the first section of the plate using tight sweeping lines that stay within that section: (1/3 for a 3-phase pattern) or (1/4 for 4-phase) of the plate. It is fine to overlap your streaks in this section. (Fig A-3)
3. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
4. Pull the loop through the previous streak (section 1) one or two times to re-inoculate the loop with cells. Now streak section 2 of the plate, avoiding section 1 after the first 1-2 streaks and trying not to overlap the streaks (Figure A-4).
5. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
6. Pull the loop through one edge of the streak in section 2 of the plate to obtain inoculum. Now streak the 3rd section of the plate.
7. Sterilize the loop and repeat 6 and 7 until you complete inoculating all sections of your plate. Incubate and look for isolated colonies that have grown from one cell (Figure A-5).

Figure A-1: Two patterns for labeling the bottom of a plate for Isolation Streak technique. The inoculating loop is sterilized between each section and inoculum is taken from the preceding section to an uninoculated section of the plate.
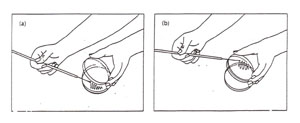
Figure A-2: Two options for aseptic transfer into a plate. (a) Streaking a plate while holding the lid ajar. Note that the lid shields the agar from airborne contamination: (b) streaking a plate while holding the bottom of the plate. Note that the agar surface faces downward, thereby minimizing contamination from the air.

Figure A-3: Pattern for isolation streaking section 1 of a plate.

Figure A-4: Illustration of isolation streak technique section 1 to section 2.

Figure A-5: An example of growth 24-72 hours after isolation streaking a plate to obtain isolated colonies.
Lab Activities
Activity 1: Aseptic Transfer Broth to Broth and Broth to Plate:
1. Obtain and label a tube of water (Tube W) and two culture tubes of LB broth. Label each of the culture tube with your initials and the date (using a marker directly on the glass). One will be labeled "E. coli" and the other "Blank". Take the stock tube of E. coli culture (Tube A-found on your bench) and using the instructions for aseptic transfer (broth to broth), transfer a loopful of the contents of Tube A to the "E. coli" labeled LB broth. Repeat the process with your negative control (Tube W), transfering a loopful of water into the culture tube labeled "Blank". Call your instructor over and ask them to assess your technique.
2. Label two destination LB plates with your initials and the date. As above, label one "E. coli" and one "Blank". Always label on the AGAR side of plate. Why is it important to not label on the cover part of the plate? Cultures on solid media are usually incubated agar side up (covers down) to prevent condensation forming on the tops and then "raining" on your growing colonies, smearing them and making it more difficult to keep them separated. In addition, if 2 or more plate covers fall off accidentally, you will be unable to match the cover to the correct plate.
Flame sterilize your loop and then insert the loop portion into the E.coli culture in Tube A. Remove the loop from the broth culture and lightly touch and drag the contaminated loop across section 1 of the destination plate (labeled "E. coli"). Follow the remaining instructions above for streaking to isolation. Repeat this process for your blank, transfering a loopful of contents from Tube W to the agar plate labeled "blank".
Come back to the lab in a few days to check on your cultures. You hope to see well isolated colonies on your plate and a turbid culture in your broth.
Activity 2: Dilutions Worksheets:
Before we can get started on our study of these microbes, we need to grow them on our defined media. One measure that microbiologists use when determining how many microbes are in an environment is called "Colony Forming Units" or CFUs. In order to calculate the total CFUs in your sample, you need to know how you have diluted the sample before plating. "Why do we dilute the sample before plating?"
Download the dilutions worksheet from the lab oncourse site and complete it to the best of your ability. We will go over the answers on Wednesday.
Activity 3: Inoculating Your Media with Drosophila Microbiome Isolates:
You each have an ice bucket in front of you with a single tube of Drosophila melanogaster flies. Your job is to first homogenize the flies using a sterile pestle and 200 ul of distilled, deionized water, and then create a dilution series, in distilled, deionized water. You will then plate two out of the 4 dilutions.
1. Remove four sterile 1.5 mL tubes at your bench (in the plastic containers with lids).
2. Label these tubes 1, 2, 3, and 4.
3. Add 0.9 mL of ddH20 to each of these tubes "what micropipette will you use?".
4. Add 0.1 mL of your original Drosophila lysate to tube 1. Cap the tube and mix by vortexing.
5. Add 0.1 mL of the liquid in tube 1 to the liquid in tube 2. Cap tube 2 and mix by vortexing.
6. Add 0.1 mL of the liquid in tube 2 to the liquid in tube 3. Cap tube 3 and mix by vortexing. Tube 3 will be our 10^3 dilution.
7. Add 0.1 mL of the liquid in tube 3 to the liquid in tube 4. Cap tube 4 and mix by vortexing. Tube 4 will be our 10^4 dilution.
8. At this point you are ready to plate your dilutions. Label two solid agar plates (MRS or LB, you will plate on both) with your initials and the dilution factor. "make sure you label the agar side!".
9. Uncap your agar plate. Take 200 ul of your dilutions (tube 3) and spot it onto the center of the agar. Using a sterile spreader, spread the liquid as evenly as possible across the surface of the agar.
10. Replace the cap and allow your sample to sit, undisturbed, agar side down, for ~2 minutes.
11. Repeat the plating and spreading for dilution tube 4 and for each of the two media types.
At the end of this exercise you will have four total plates - two dilutions on both MRS and LB.
More Tools & Techniques of Microbiologists
Use and Calibration of Micropipets
How to Use a Micropipettor
The following website Using a Micropipette has more detailed information about micropipette use.
- Decide which of your micropipets is appropriate for the volume you want to measure and dispense.
- Adjust the volume dial to the appropriate volume, recognizing that your P1000 must have a zero added to the bottom of the volume display boxes and the P20 has a decimal between the bottom and second volume display boxes.
- Firmly seat a new micropipet tip of appropriate size on the micropipets.
- Depress with the thumb plunger to the first stop and hold the pipettor in this depressed position (DO NOT depress fully).
- Dip the micropipet into the solution far enough to account for the volume that will be withdrawn but not so far as to immerse the micropipet barrel.
- Gradually release the plunger, drawing fluid into the tip without forming bubbles.
- Carefully slide the micropipet tip along the side of the tube to remove any unwanted droplets of fluid sticking to the tip's surface.
- Expel the fluid into the desired container by touching the micropipet tip to the inside surface of the container and slowly depressing FULLY the plunger.
- Continue to hold the plunger in the fully depressed position as you remove the micropipet from the container.
- Eject the tip by pressing the eject button (if your micropipet has one) into an appropriate place (only tips that have been contaminated with microorganisms need to be ejected into an autoclave bag).
Don'ts in Using Micropipets
- DO NOT force or rotate the volume adjustment knob past the upper or lower ranges specified on the top of the micropipet.
- DO NOT use a micropipet without a tip since the precision piston that measures the volume can be ruined.
- DO NOT put the micropipet down on your bench with a filled tip since fluid can run back into the precision piston.
- DO NOT allow the micropipet plunger to snap back after fluid is either dispensed or drawn.
- DO NOT immerse the micropipet barrel into fluid.
- DO NOT flame the micropipet tip.
Activity 4: Protocol for Micropipet Calibration
1. To calibrate your P1000, P 200, and P 20 micropipets, label 6 microfuge tubes (1-6) and weigh them on a top loading balance. Don't forget to tare the balance. Record the weights in a table in your lab notebook, like the one below.
2. Using the information in the table below, pipet the specified volumes into the pre-weighed microfuge tubes prepared in step 1 and then reweigh the tubes. Record all weights.
3. Calculate the weight of the water in grams by substracting the dry weight from the weight of the tubes with water. Note that 1000 microliters of water should weigh exactly 1 gram at room temperature.
4. If the water in any tube weighs more or less than 1 gram, repeat that tube. Ask your instructor to check your pipetting technique if your calibration continues to be off after several repeated attempts.
| Tube # | Pre-weight | Tube Vol. in µl using P20 |
Vol. in µl using P200 |
Vol. in µl using P1000 |
Weight of water grams |
|---|---|---|---|---|---|
| 1 | _____ | 10 | 0 | 990 | ____ |
| 2 | _____ | 0 | 100 | 900 | ____ |
| 3 | _____ | 20 | 175 | 805 | _____ |
| 4 | _____ | 2 | 88 | 910 | ______ |
| 5 | _____ | 0 | 200 x 5 | 0 | _____ |
| 6 | _____ | 20 x 5 | 0 | 900 | _____ |
CLEAN UP
1. Please put your inoculated plates and broth in the 30C incubator in the back of the room.
2. When done with liquid cultures, remove tape from all glass tubes. Then place the glass tubes with caps in the metal containers by the door to the room. Do not discard the contents of the tubes. When done with
plates, put them into the autoclave bags next to the laminar flow hoods.
3. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
4. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the containers to be autoclaved.
5. Place all your equipment back where you found it at the beginning of the day.
6. Place your worksheet within your binder and leave it at your bench, in your drawer for your instructor to collect.
7. Wash your hands.
