M465:Interactions: Difference between revisions
| Line 35: | Line 35: | ||
Beginning with Isolate #2, inoculate a second 50 μl of each of your isolates into the column wells B1, C1, etc. (indicated by the green color). | Beginning with Isolate #2, inoculate a second 50 μl of each of your isolates into the column wells B1, C1, etc. (indicated by the green color). | ||
<BR> | <BR> | ||
Add | Add 50 μL of appropriate broth to each of the wells containing your isolates (row wells A1-A8 and column wells B1-H1) | ||
<BR> | <BR> | ||
Revision as of 09:37, 3 February 2014
Antagonistic and Mutualistic Interactions
*NOTE: You must remember to set up fresh broth cultures for your isolates 1-3 days before lab to do this test!
The microbial community living in your environments is a complex one with many different microorganisms. As is true of any environment, these microbes interact with each other - both functionally and physically. Do selected bacteria from your community help each other or harm each other while trying to find a niche in your community? Today, you will try to answer that question by testing your cultured isolates for examples of mutualism or antagonism (co-operation or competition)by culturing them in controlled communities. Some of these bacteria may prevent the growth of others through the production of chemical inhibitors; others might promote the growth of their neighbors by producing metabolites that are needed. We are going to look for both positive and negative interactions.
PREPARING THE ISOLATES:
You will inoculate 50 µl of log phase (young culture) isolate grown in fresh broth into the assigned well(s). Once again try to control for similar numbers of organisms in your inoculum by starting with normalized optical density measurements (remember to use the OD600_calcs.xls spreadsheet!)
Interaction Assay Set Up
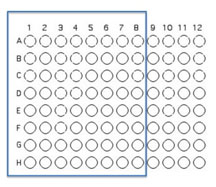
You will use 64 of the wells on a 96 well plate for this assay. Each of you will use all of your unique isolates to test for interactions. Use Excel to keep track of which organisms that will be inoculated into each well as described and illustrated below.
FOLLOW THE TEMPLATE CAREFULLY!!!!!! It is easy to get this inoculation messed up, but don't!

Transfer 50 μL of each of the isolates to be tested into the illustrated row of wells (A1 is Isolate 1, A2 is Isolate #2 etc through A8, should you have that many)
Beginning with Isolate #2, inoculate a second 50 μl of each of your isolates into the column wells B1, C1, etc. (indicated by the green color).
Add 50 μL of appropriate broth to each of the wells containing your isolates (row wells A1-A8 and column wells B1-H1)
Gently move the 96 well plate in a circular motion to mix.

Transfer 10 μl of the contents of the 7 wells - A2 (containing isolate #2 etc) through A8 to the empty wells in each column as indicated by the yellow arrows. You will need to remove the first tip from a multichannel pipette. If you are using the multichannel pipette, be sure that you work slowly and check that each pipette tip is evenly filled. You may need to tighten the tips by hand, if so be sure to only touch the part of the tip that sits on the multichannel pipette, you wouldn't want to contaminate your wells with human organsisms!
Transfer 10 μl of the contents of wells A1 (containing isolate #1 etc) through H1 to each well in the row as indicated by the red arrows.
Again gently mix the contents of the well by moving the plate in gentle circles.
At the end of these transfers, measure the optical density in each well using the plate reader.
This same set of wells will be used to inoculate an agar plate. Label an agar plate (with media appropriate to your isolates) with your name and the class name and date. Using a multichannel pipette, take 5 ul of your mixed cultures from your wells and inoculate the surface of the agar. Do not punch a hole in the agar using your pipette tip! Be careful! These drops of fluid will evaporate in the hood if you leave the lid off your plate for a few minutes.
Place both your 96-well plate and the agar plate at 37C to grow under appropriate atmosphere. Take measurements on the optical density daily to confirm growth inhibition or stimulation. Look at your mixed cultures which you have spotted onto the agar plate after 2 days of growth. Can you see any interesting patterns in the growth?