M465:StreakingtoIsolation: Difference between revisions
| Line 1: | Line 1: | ||
=='''Calculation the number of CFUs in your sample | =='''Calculation the number of CFUs in your sample'''== | ||
When we last met, you plated out your honey bee sample onto media and learned how to calculate the total number of bacteria in the original sample using our dilution series worksheet. Today you will count to get one kind of enumeration of the microorganisms in your sample <BR><BR> | When we last met, you plated out your honey bee sample onto media and learned how to calculate the total number of bacteria in the original sample using our dilution series worksheet. Today you will count to get one kind of enumeration of the microorganisms in your sample <BR><BR> | ||
| Line 13: | Line 13: | ||
Why did we perform so many dilutions when we set up our plate count?<BR> | Why did we perform so many dilutions when we set up our plate count?<BR> | ||
Why should you have only one dilution with 30-300 colonies?<BR> | Why should you have only one dilution with 30-300 colonies?<BR> | ||
==Protocol for Streaking for Isolation== | ==Protocol for Streaking for Isolation== | ||
Revision as of 13:07, 13 January 2014
Calculation the number of CFUs in your sample
When we last met, you plated out your honey bee sample onto media and learned how to calculate the total number of bacteria in the original sample using our dilution series worksheet. Today you will count to get one kind of enumeration of the microorganisms in your sample
Calculating the number of colony forming units (CFU) in your original sample
The original sample given to you was 200 uL total volume. Keep that in mind for your calculations below!
Examine the plates at each of the two dilutions you plated and find one dilution with between 30 and 300 colonies. You will not assess plates with well over 300 colonies or under 30; they should be designated as "invalid" in your worksheet. Count all the surface and subsurface colonies on the plates (those with 30-300 colonies). Divide the total number of colonies counted by the amount of inoculum plated times the dilution factor of that plate to obtain the number of bacteria in your original sample.
Record the number of CFU/ total sample in your lab notebook and enter your data on the course spreadsheet on the instructor's computer at the front of the lab. Calculate the average and standard deviation for all the plates for your media. We will refer to this number as the average MRS or LB colony forming units/sample.
Things to Consider:
Why did we perform so many dilutions when we set up our plate count?
Why should you have only one dilution with 30-300 colonies?
Protocol for Streaking for Isolation
During our last meeting you plated honey bee gut isolates onto either MRS or LB agar media. These colonies have grown up at 37C, under anaerobic conditions. In order to work with your bacteria for this part of the semester, we need to ensure that you have well isolated, individual colonies. For that reason, we will pick five colonies on your original plates and re-streak them for isolation (protocol below).
Your agar plates can be streaked using a three-phase or a four phase pattern (Figure A-1). YouTube Demo | http://www.youtube.com/watch?v=foVPx5L3dKY&feature=PlayList&p=AB6C2197E755C3EC&index=5
The handling of the plate can be accomplished in a number of ways, all of which attempt to minimize possible contamination by either keeping the lid over the plate or by keeping the plate upside down (Figure A-2).
Isolation streak technique: from plate to plate.
1. Follow the protocol for aseptic transfer you learned last Monday. If using a loop, sterilize it in the incinerator and allow the loop to cool for a few seconds. Not allowing any cap, lid, or plug to leave your hand, touch your loop to a pure colony of bacteria on a streak plate.
2. Using the loop, streak the first section of the plate using tight sweeping lines that stay within that section: (1/3 for a 3-phase pattern) or (1/4 for 4-phase) of the plate. It is fine to overlap your streaks in this section. (Fig A-3)
3. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
4. Pull the loop through the previous streak (section 1) one or two times to re-inoculate the loop with cells. Now streak section 2 of the plate, avoiding section 1 after the first 1-2 streaks and trying not to overlap the streaks (Figure A-4).
5. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
6. Pull the loop through one edge of the streak in section 2 of the plate to obtain inoculum. Now streak the 3rd section of the plate.
7. Sterilize the loop and repeat 5 and 6 until you complete inoculating all sections of your plate. Incubate and look for isolated colonies that have grown from one cell (Figure A-5).

Figure A-1: Two patterns for labeling the bottom of a plate for Isolation Streak technique. The inoculating loop is sterilized between each section and inoculum is taken from the preceeding section to an uninoculated section of the plate.
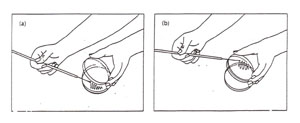
Figure A-2: Two options for aseptic transfer into a plate. (a) Streaking a plate while holding the lid ajar. Note that the lid shields the agar from airborne contamination: (b) streaking a plate while holding the bottom of the plate. Note that the agar surface faces downward, thereby minimizing contamination from the air.

Figure A-3: Pattern for isolation streaking section 1 of a plate.

Figure A-4: Illustration of isolation streak technique section 1 to section 2.

Figure A-5: An example of growth 24-72 hours after isolation streaking a plate to obtain isolated colonies.