Molecular Recognition Laboratorium: Difference between revisions
Victor Tapia (talk | contribs) |
|||
| (97 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Image:MolRec-Logo.jpg| | [[Image:MolRec-Logo.jpg|600px]] | ||
<div style="padding: 10px; color: # | <div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | ||
| Line 9: | Line 10: | ||
CHARITÉ - UNIVERSITÄTSMEDIZIN BERLIN<br> | CHARITÉ - UNIVERSITÄTSMEDIZIN BERLIN<br> | ||
{{hide| | {{hide| | ||
[[Image:Molrec placeholder.png|112px|right]] | |||
Hessische Str. 3-4 | Hessische Str. 3-4 | ||
D-10115 Berlin, Germany | D-10115 Berlin, Germany | ||
| Line 17: | Line 19: | ||
web charite.de | web charite.de | ||
}} | }} | ||
last change on 01.04.2010 | |||
<br> | <br> | ||
</div><br> | </div><br> | ||
==Group Leader== | ==Group Leader== | ||
Rudolf Volkmer {{hide| | <div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | ||
Rudolf Volkmer {{hide| | |||
[[Image:Molrec placeholder.png|112px|right]] | |||
Tel.: +49 30 450 524 267 | Tel.: +49 30 450 524 267 | ||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: rve(at)charite.de | E-Mail: rve(at)charite.de | ||
}} | }} | ||
<br> | <br> | ||
</div><br> | |||
==Group Members== | ==Group Members== | ||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | |||
* | *Magdalena Czuban, Dipl. Biol. {{hide| | ||
Tel.: +49 30 450 | [[Image:Molrec placeholder.png|112px|right]] | ||
Tel.: +49 30 450 xxx xxx | |||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: | E-Mail: x(at)charite.de | ||
}} | }} | ||
* | *Mercedes Gonzáles, Studentin {{hide| | ||
Tel.: +49 30 450 | [[Image:Molrec placeholder.png|112px|right]] | ||
Tel.: +49 30 450 xxx xxx | |||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: | E-Mail: x(at)charite.de | ||
}} | }} | ||
* | *Anja Heiduk, Dipl. Biol. {{hide| | ||
Tel.: +49 30 450 | [[Image:Molrec placeholder.png|112px|right]] | ||
Tel.: +49 30 450 xxx xxx | |||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: | E-Mail: x(at)charite.de | ||
}} | }} | ||
* | *Simone Jagdhuber, Studentin {{hide| | ||
Tel.: +49 30 450 | [[Image:Molrec placeholder.png|112px|right]] | ||
Tel.: +49 30 450 xxx xxx | |||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: | E-Mail: x(at)charite.de | ||
}} | }} | ||
* | *Ines Kretzschmar, TA {{hide| | ||
Tel.: +49 30 | [[Image:Molrec placeholder.png|112px|right]] | ||
Fax: +49 30 | Tel.: +49 30 x | ||
E-Mail: | Fax: +49 30 x | ||
E-Mail: x(at)charite.de | |||
}} | }} | ||
* | *Christiane Landgraf, TA {{hide| | ||
[[Image:Molrec placeholder.png|112px|right]] | |||
Tel.: +49 30 450 524 xxx | |||
Tel.: +49 30 450 524 | |||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: chl(at)charite.de | E-Mail: chl(at)charite.de | ||
}} | }} | ||
* | *Eric Moinet, Student {{hide| | ||
Tel.: +49 30 450 524 | [[Image:Molrec placeholder.png|112px|right]] | ||
Tel.: +49 30 450 524 xxx | |||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: | E-Mail: x(at)charite.de | ||
}} | }} | ||
* | *[http://openwetware.org/wiki/User:Victor_Tapia Víctor Tapia] - Doktorand {{hide| | ||
[[Image:Molrec placeholder.png|112px|right]] | |||
Tel.: +49 30 450 524 285 | |||
Fax: +49 30 450 524 942 | Fax: +49 30 450 524 942 | ||
E-Mail: | E-Mail: victor.tapia(at)charite.de; ve.tapia.m(at)gmail.com | ||
}} | |||
<br> | |||
</div><br> | |||
}} | |||
==Research interest== | |||
The AG Volkmer emerges from a strong training in peptide chemistry and cultivates its expertise in the synthesis and preparation of peptide/peptoide probes, either as core service or to meet the demands of our own research in cellular biology and molecular medicine. | |||
The objectives of our research principally spread around three main topics: profiling the binding specificity of protein recognition modules, analyzing the diagnostic potential peptide-based analyte capture assays, and exploring the potential of peptide/peptoide probes to define or modulate specific therapeutic strategies. | |||
<br> | |||
===Profiling the binding specificity of protein recognition modules=== | |||
[[Image:Drawing.png|400px]] | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | |||
Protein recognition modules (PRM) are non-catalytic domains of protein structure dedicated to read molecular motifs of primary structure and post-translational modifications of proteins. Reading or recognition is not restricted to protein motifs, as shown by the emerging field of epigenetics. It is clear that methylation and other signatures on DNA are also recognized by modular structures of nuclear factors. | |||
The tinkering of evolution has repeatedly duplicated and diverged different structural modules resulting in several homology families with some degree of functional conservation. This is observed as regular expressions of short linear motifs that can be recognized by a PRM family and by specific affinity traits of individual PRMs. | |||
We aim to identify key events in cellular processes of information reading and transduction. Success in such aim translates to engineering congruent interaction networks, complementing drug efficiency and designing new therapeutic strategies, as has been shown by our group with several domains, i.e. WW domains in X-linked intellectual disorders and mechanosensing, SH3 domains in endocytosis, as well as PDZ domains in cystic fibrosis. | |||
</div> | |||
<br> | |||
===Analyzing the diagnostic potential of peptide-based analyte capture assays=== | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | |||
We intend to focus away from single biomarker for diagnosis and use either validated collections or agnostic collections of peptide probes to analyze complex biological samples. The former approach relies on the use of peptide probes known to interact with one or multiple target biomarkers for a specific pathology, the later one relies on extensive stochastic peptide probes to capture eventually unknown analytes in the sample that can be used as a diagnostic pattern of detection signals upon a multiplex binding assay. | |||
The development of these approaches can be estimated from reports of our group and cooperation partners inside as well as outside RCIS. These reports show the difficulties and potential of the use of peptide probes to analyze blood samples and diagnose hypersensitivity, autoimmune responses, and immunological responses to infection. | |||
</div> | |||
<br> | |||
===Exploring the therapeutic potential of peptide/peptoid probes=== | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | |||
The flag ship of this research venue derives from the unique expertise for generating immobilized but C-terminal exposed peptide probes and accurate experience with PDZ specificity profiles. A peptide probe has been design to selectively inhibit the CFTR–CAL interaction —relevant in cystic fibrosis— without affecting the biologically relevant PDZ competitors NHERF1 and NHERF2. | |||
In this venue of research we also explore the potential of cell penetrating peptides to be applied as vector to transport drug probes, such as the CFTR-CAL inhibitor, across the cell membrane. | |||
</div> | |||
<br> | <br> | ||
===Technological Development of the Peptide Array Technologies=== | ===Technological Development of the Peptide Array Technologies=== | ||
by ''Victor Tapia'' <br> | by ''Victor Tapia'' <br> | ||
<br> | |||
---- | |||
[[image:Macro_to_microarrays_of_peptides.png|400px]] | |||
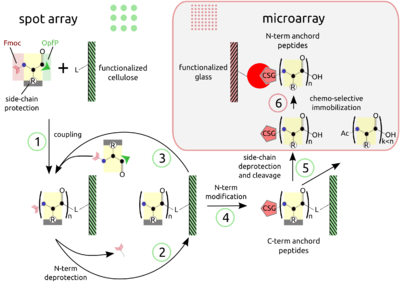
The basic point of this technology is the simultaneous display of a systematic collection of peptides on a planar support, on which numerous bimolecular interaction assays can be carried out under homogeneous conditions. | The combination of SPOT peptide synthesis (figure A, steps 1 to 4) with | ||
appropriate immobilization techniques on glass supports (figure A, steps | |||
5 and 6) is wide spread. The SPOT technology provides low-scale but | |||
high-throughput synthesis, while immobilization of pre-synthesized | |||
peptides offers the benefit of a "chemical" purification step and | |||
flexible array design. Additionally, the glass support is compatible | |||
with fluorescence detection and | |||
offers the possibility to miniaturize binding assays. Beyond economy, | |||
the later point is essential for quantitative measurements at the | |||
steady-state of binding activity, as has been described [Ekins 1998] and | |||
can be proven by the mass-action law. <br> | |||
---- | |||
<br> | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | |||
The basic point of this technology is the simultaneous display of a <br> | |||
systematic collection of peptides on a planar support, on which numerous <br> | |||
bimolecular interaction assays can be carried out under homogeneous <br> | |||
conditions. <br> | |||
<br> | |||
---- | ---- | ||
PEPTIDE ARRAYS IN THE ADVANCEMENT OF PEPTIDE SYNTHESIS<br> | |||
<br> | |||
* The development of solid-phase peptide synthesis (SPPS) by Bruce Merrifield [Gutte and Merrifield, 1969; Merrifield, 1965] and adaptions of this procedure [Fields and Noble, 1990] set the chemical ground for innovative technologies to follow. <br> | |||
* The development of the “Pin” method by H. Geysen [Geysen, et al., 1984] introduces the array format to peptide synthesis. <br> | |||
* Definitive establishment of peptide arrays came along with the development of the SPOT synthesis by Roland Frank [Frank, 1992; Frank, 2002] which simplified chemical synthesis of peptide arrays to the addressable deposition of reagents on a cellulose sheet. <br> | |||
<br> | |||
Modern peptide synthesis approaches and <br> | |||
molecular biology make peptides accessible in a high degree of <br> | |||
structural diversity. The two greatest drawbacks of synthetic peptide <br> | |||
arrays are peptide length, with a quality threshold between 30 and 50 <br> | |||
amino-acids, as well as the restriction to linear motives, since the <br> | |||
mimicry of nonlinear motives with linear peptide constructs is still <br> | |||
under development [Goede, et al., 2005]. <br> | |||
<br> | |||
---- | ---- | ||
PEPTIDE ARRAYS IN THE ADVANCEMENT OF BINDING ASSAY SYSTEMS <br> | |||
<br> | <br> | ||
Since the 90s a major aspect of development to achieve the required <br> | |||
sensitivities to analyse biological samples has been the miniaturization <br> | |||
of analytical devices [Ekins, 1998]. It is important to note that <br> | |||
miniaturization is not only a matter of high-throughput and economy. <br> | |||
Miniaturization is an essential factor that should provide saturation of <br> | |||
binding sites under low analyte concentrations without significantly <br> | |||
altering its bulk (or ambient) concentration upon capturing [Ekins, et <br> | |||
al., 1990; Ekins, 1989; Joos, et al., 2002; Templin, et al., 2002]. | |||
* In this sense, the first application of a peptide microarray device in 1991, anticipating even the application of cDNA arrays, achieved already the impressive feature density of about 1024 peptides in 1.6 cm2 by means of in situ light-directed parallel synthesis [Fodor, et al., 1991]. | |||
Several methods available to generate peptide arrays on planar solid surfaces offer a range between... | |||
* 16 peptides per cm2, in the case of SPOT macroarrays [Reimer, et al., 2002; Schutkowski, et al., 2004], | |||
* to 2000-4000 peptides in 1.5 cm2, in the case of microarrays generated by digital photolithography [El Khoury, et al., 2007; Gao, et al., 2004; Pellois, et al., 2000; Pellois, et al., 2002]. | |||
<br> | <br> | ||
[ | ---- | ||
SOURCES: For references to citations above see | |||
* Tapia, V.E., Ay, B., Volkmer, R., 2009. Exploring and Profiling Protein Function with Peptide Arrays, in: Marina Cretich, Chiari, M. (Eds.), Peptide Microarrays. Humana Press, Totowa, NJ, pp. 3–17. | |||
* Tapia, VE & R Volkmer, 2009. Steady State Analysis of Peptide Array-based Binding Assays [https://www.openwetware.org/images/e/e7/Steady-state_binding_asssays.pdf] | |||
</div> | |||
<br> | <br> | ||
== | == Who's visiting == | ||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 600px"> | |||
start on 18 Mar 2010 | |||
== | <html> | ||
<a href="http://www2.clustrmaps.com/counter/maps.php?url=http://openwetware.org/wiki/Molecular_Recognition_Laboratorium" id="clustrMapsLink"><img src="http://www2.clustrmaps.com/counter/index2.php?url=http://openwetware.org/wiki/Molecular_Recognition_Laboratorium" style="border:0px;" alt="Locations of visitors to this page" title="Locations of visitors to this page" id="clustrMapsImg" onerror="this.onerror=null; this.src='http://clustrmaps.com/images/clustrmaps-back-soon.jpg'; document.getElementById('clustrMapsLink').href='http://clustrmaps.com';" /> | |||
</a> | |||
</html> | |||
</div> | |||
==Science in the city: A small series about the everyday life of scientists in berlin== | |||
'''FOLLOW ME''' [http://openwetware.org/wiki/User:Victor_Tapia#Science_in_the_city >>>>] | |||
Latest revision as of 06:11, 13 November 2014
Contact Information
Molecular Recognition Laboratorium,
Institute für medizinische Immunologie
CHARITÉ - UNIVERSITÄTSMEDIZIN BERLIN

Hessische Str. 3-4 D-10115 Berlin, Germany phone +49-30-450 524092 fax +49-30-450 524942 mail annette.hayungs@charite.de web charite.de
last change on 01.04.2010
Group Leader
Group Members
- Magdalena Czuban, Dipl. Biol.
- Mercedes Gonzáles, Studentin
- Anja Heiduk, Dipl. Biol.
- Simone Jagdhuber, Studentin
- Ines Kretzschmar, TA
- Christiane Landgraf, TA
- Eric Moinet, Student
- Víctor Tapia - Doktorand

Tel.: +49 30 450 524 285 Fax: +49 30 450 524 942 E-Mail: victor.tapia(at)charite.de; ve.tapia.m(at)gmail.com
Research interest
The AG Volkmer emerges from a strong training in peptide chemistry and cultivates its expertise in the synthesis and preparation of peptide/peptoide probes, either as core service or to meet the demands of our own research in cellular biology and molecular medicine.
The objectives of our research principally spread around three main topics: profiling the binding specificity of protein recognition modules, analyzing the diagnostic potential peptide-based analyte capture assays, and exploring the potential of peptide/peptoide probes to define or modulate specific therapeutic strategies.
Profiling the binding specificity of protein recognition modules
Protein recognition modules (PRM) are non-catalytic domains of protein structure dedicated to read molecular motifs of primary structure and post-translational modifications of proteins. Reading or recognition is not restricted to protein motifs, as shown by the emerging field of epigenetics. It is clear that methylation and other signatures on DNA are also recognized by modular structures of nuclear factors. The tinkering of evolution has repeatedly duplicated and diverged different structural modules resulting in several homology families with some degree of functional conservation. This is observed as regular expressions of short linear motifs that can be recognized by a PRM family and by specific affinity traits of individual PRMs. We aim to identify key events in cellular processes of information reading and transduction. Success in such aim translates to engineering congruent interaction networks, complementing drug efficiency and designing new therapeutic strategies, as has been shown by our group with several domains, i.e. WW domains in X-linked intellectual disorders and mechanosensing, SH3 domains in endocytosis, as well as PDZ domains in cystic fibrosis.
Analyzing the diagnostic potential of peptide-based analyte capture assays
We intend to focus away from single biomarker for diagnosis and use either validated collections or agnostic collections of peptide probes to analyze complex biological samples. The former approach relies on the use of peptide probes known to interact with one or multiple target biomarkers for a specific pathology, the later one relies on extensive stochastic peptide probes to capture eventually unknown analytes in the sample that can be used as a diagnostic pattern of detection signals upon a multiplex binding assay. The development of these approaches can be estimated from reports of our group and cooperation partners inside as well as outside RCIS. These reports show the difficulties and potential of the use of peptide probes to analyze blood samples and diagnose hypersensitivity, autoimmune responses, and immunological responses to infection.
Exploring the therapeutic potential of peptide/peptoid probes
The flag ship of this research venue derives from the unique expertise for generating immobilized but C-terminal exposed peptide probes and accurate experience with PDZ specificity profiles. A peptide probe has been design to selectively inhibit the CFTR–CAL interaction —relevant in cystic fibrosis— without affecting the biologically relevant PDZ competitors NHERF1 and NHERF2. In this venue of research we also explore the potential of cell penetrating peptides to be applied as vector to transport drug probes, such as the CFTR-CAL inhibitor, across the cell membrane.
Technological Development of the Peptide Array Technologies
by Victor Tapia
The combination of SPOT peptide synthesis (figure A, steps 1 to 4) with
appropriate immobilization techniques on glass supports (figure A, steps
5 and 6) is wide spread. The SPOT technology provides low-scale but
high-throughput synthesis, while immobilization of pre-synthesized
peptides offers the benefit of a "chemical" purification step and
flexible array design. Additionally, the glass support is compatible
with fluorescence detection and
offers the possibility to miniaturize binding assays. Beyond economy,
the later point is essential for quantitative measurements at the
steady-state of binding activity, as has been described [Ekins 1998] and
can be proven by the mass-action law.
The basic point of this technology is the simultaneous display of a
systematic collection of peptides on a planar support, on which numerous
bimolecular interaction assays can be carried out under homogeneous
conditions.
PEPTIDE ARRAYS IN THE ADVANCEMENT OF PEPTIDE SYNTHESIS
- The development of solid-phase peptide synthesis (SPPS) by Bruce Merrifield [Gutte and Merrifield, 1969; Merrifield, 1965] and adaptions of this procedure [Fields and Noble, 1990] set the chemical ground for innovative technologies to follow.
- The development of the “Pin” method by H. Geysen [Geysen, et al., 1984] introduces the array format to peptide synthesis.
- Definitive establishment of peptide arrays came along with the development of the SPOT synthesis by Roland Frank [Frank, 1992; Frank, 2002] which simplified chemical synthesis of peptide arrays to the addressable deposition of reagents on a cellulose sheet.
Modern peptide synthesis approaches and
molecular biology make peptides accessible in a high degree of
structural diversity. The two greatest drawbacks of synthetic peptide
arrays are peptide length, with a quality threshold between 30 and 50
amino-acids, as well as the restriction to linear motives, since the
mimicry of nonlinear motives with linear peptide constructs is still
under development [Goede, et al., 2005].
PEPTIDE ARRAYS IN THE ADVANCEMENT OF BINDING ASSAY SYSTEMS
Since the 90s a major aspect of development to achieve the required
sensitivities to analyse biological samples has been the miniaturization
of analytical devices [Ekins, 1998]. It is important to note that
miniaturization is not only a matter of high-throughput and economy.
Miniaturization is an essential factor that should provide saturation of
binding sites under low analyte concentrations without significantly
altering its bulk (or ambient) concentration upon capturing [Ekins, et
al., 1990; Ekins, 1989; Joos, et al., 2002; Templin, et al., 2002].
- In this sense, the first application of a peptide microarray device in 1991, anticipating even the application of cDNA arrays, achieved already the impressive feature density of about 1024 peptides in 1.6 cm2 by means of in situ light-directed parallel synthesis [Fodor, et al., 1991].
Several methods available to generate peptide arrays on planar solid surfaces offer a range between...
- 16 peptides per cm2, in the case of SPOT macroarrays [Reimer, et al., 2002; Schutkowski, et al., 2004],
- to 2000-4000 peptides in 1.5 cm2, in the case of microarrays generated by digital photolithography [El Khoury, et al., 2007; Gao, et al., 2004; Pellois, et al., 2000; Pellois, et al., 2002].
SOURCES: For references to citations above see
- Tapia, V.E., Ay, B., Volkmer, R., 2009. Exploring and Profiling Protein Function with Peptide Arrays, in: Marina Cretich, Chiari, M. (Eds.), Peptide Microarrays. Humana Press, Totowa, NJ, pp. 3–17.
- Tapia, VE & R Volkmer, 2009. Steady State Analysis of Peptide Array-based Binding Assays [1]
Who's visiting
start on 18 Mar 2010
<html> <a href="http://www2.clustrmaps.com/counter/maps.php?url=http://openwetware.org/wiki/Molecular_Recognition_Laboratorium" id="clustrMapsLink"><img src="http://www2.clustrmaps.com/counter/index2.php?url=http://openwetware.org/wiki/Molecular_Recognition_Laboratorium" style="border:0px;" alt="Locations of visitors to this page" title="Locations of visitors to this page" id="clustrMapsImg" onerror="this.onerror=null; this.src='http://clustrmaps.com/images/clustrmaps-back-soon.jpg'; document.getElementById('clustrMapsLink').href='http://clustrmaps.com';" /> </a> </html>
Science in the city: A small series about the everyday life of scientists in berlin
FOLLOW ME >>>>