Payne Lab: Difference between revisions
Edwin Shin (talk | contribs) No edit summary |
Edwin Shin (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:PayneBanner.jpg| | [[Image:PayneBanner.jpg|820px|center]] | ||
<div style="padding: 10px; color: #ffffff; background-color: #000; width: 800px; margin: auto;"> | <div style="padding: 10px; color: #ffffff; background-color: #000; width: 800px; margin: auto;"> | ||
<center> | <center> | ||
Revision as of 14:45, 20 February 2012

Imaging Chemical Reactions in Living Cells
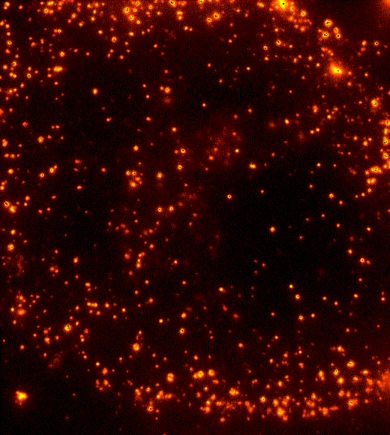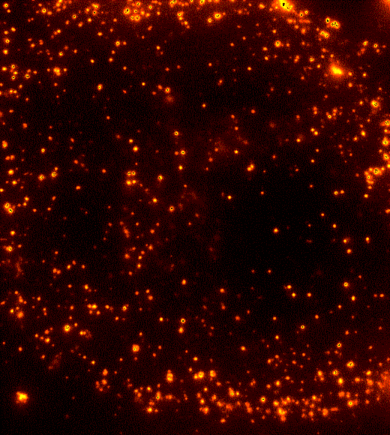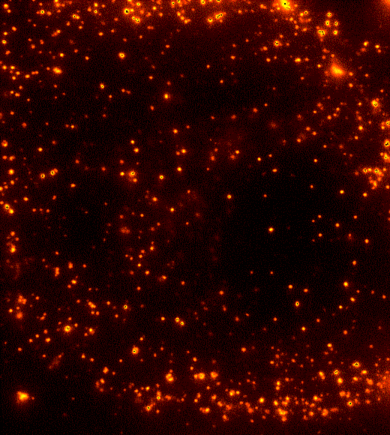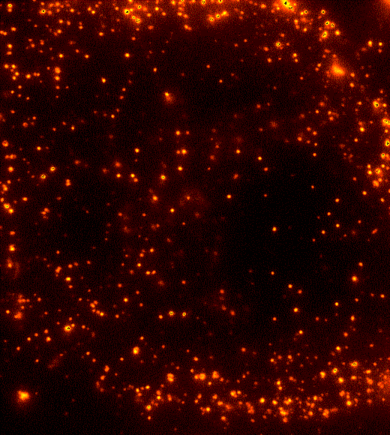
Cells are dynamic environments that use carefully regulated mechanisms to maintain function and health. One example of this is the vesicle-mediated transport of lipids (shown to the right). Each bright spot shows a single vesicle as it transports lipids through the cell. Each step of this process; internalization, transport in the vesicle, and enzymatic degradation of the lipids, is controlled by chemical reactions within the cell. Understanding these dynamic processes requires a method that will provide both spatial and temporal information-the ability to watch each step as it occurs. To obtain this information the Payne Lab uses fluorescence microscopy to directly probe intracellular dynamics.
Intracellular degradation of extracellular cargo
Cells control the chemical reactions responsible for the utilization of nutrients, replication of viruses, and regulation of receptors through the localization of substrates and enzymes within distinct vesicles that are actively transported through the cell. We are especially interested in the reactions that result from the interaction of substrate-containing vesicles with enzyme-containing vesicles. We are using two-color single particle tracking to address this question in standard cell lines and in a cellular model of the blood-brain barrier.
Nanoparticle-cell interactions
Nanoparticles have important biomedical applications ranging from the treatment of human disease with gene therapy to understanding basic cellular functions with fluorescent probes. For these applications to be fully realized it is necessary to understand how nanoparticles interact with cells. The Payne Lab is especially interested in the cellular binding and internalization of nanoparticles in the presences of extracellular proteins. A combination of advanced microscopy techniques and calorimetry is being used to understand these fundamental interactions.
New methods for live cell imaging
While recent developments in fluorescence microscopy make it possible to image many of the dynamic events that are essential to cellular function, new methods are necessary to observe the dynamics of single molecules inside living cells. Imaging within live cells is difficult as the emission from fluorescent probes competes with the autofluorescence of the cell. The Payne Lab is developing new optical techniques for quantitative cellular imaging. Optical methods of interest include nanometer-level imaging, spectroscopic single-particle tracking, and multiphoton total internal reflection microscopy.