Physics307L:People/Smith/Notebook
Lab Summaries
Lab 1: Oscilloscope Lab
- See lab manual for what this lab was about.
- See my Lab Notebook entry for my remarks and recorded data.
Data
Reported value for fall time using AC coupling:
- The Digital Storage Oscilloscope (DSO) has a built in function to measure "fall-time". It reported 239.6ms to 241.0ms. This was believed to be incorrect.
- Using the cursors in the DSO interface to measure the time between the peak value and 10% of that value and using a frequency of between 2.343Hz and 2.430Hz reports a value of 58.00 ms.
- Subsequent measurements using this method after changing the frequency on the frequency generator:
- Frequency: 4.798Hz - 5.061Hz, fall time: 57.00ms.
- Frequency: 7.257Hz - 7.262Hz, fall time: 55.00ms.
- Subsequent measurements using this method after changing the frequency on the frequency generator:
Note: The DSO displays these times in integer values of milliseconds. Therefore, the precision of these measurements is necessarily limited. I suspect the way the DSO rounds these numbers makes the screen display, for instance, 55.00ms for any measurement between 54.5ms and 55.5ms.
What did you learn?
I had little previous experience with oscilloscopes before this lab. I was able to familiarize myself with the the way oscilloscopes are hooked up, how they used to work (scanning CRT displays, etc), and to interact with the modern DSO we used (it has an interface similar to an ATM machine, with many useful functions able to be selected and displayed using the buttons on its face.) I also learned a bit about the concept of AC coupling, which I had never heard of before. I also wrote my first lab notebook entry in the wiki format, which was very straightforward.
What could make the lab better next year?
Well, I saw a video online of someone who interfaced an oscilloscope with a soundcard on his PC and was able to do very, very impressive things; he wrote things on the screen, had very neat patterns and effects. I don't think we should do all that, but it's an interesting concept, and it might be worth explaining how someone would be able to do all that. You can see the video here.
Lab 2: Balmer Series
Purpose
The objective of this lab is to experimentally determine the value of the Rydberg Constant by measuring the atomic emission spectral wavelengths of hydrogen and the hydrogen-like element deuterium (which is hydrogen with an extra neutron thrown in).
Data
See the data collected section of my lab notebook entry.
I used Excel to analyze the data (you can see my Excel spreadsheet here). I used our measurements of the wavelengths of the atomic emission spectra of hydrogen and of deuterium to calculate the measured Rydberg Constant (they are, believe it or not, slightly different despite the name "Rydberg Constant"). I also calculate the expected Rydberg Constant for hydrogen and deuterium by using fundamental physical constants, and compared these with our measured values. They are reported below:
| Expected Rydberg Constants | Avg. Measured Rydberg Constants | Percent Difference | ||
|---|---|---|---|---|
| Hydrogen | Clockwise | 1.09677286888108E+07 [math]\displaystyle{ m^{-1} }[/math] | 1.09797802016246E+07 [math]\displaystyle{ m^{-1} }[/math] | -0.1099% |
| Counterclockwise | 1.09677286888108E+07 [math]\displaystyle{ m^{-1} }[/math] | 1.09583397614656E+07 [math]\displaystyle{ m^{-1} }[/math] | 0.0856% | |
| Deuterium | Clockwise | 1.09647449477397E+07 [math]\displaystyle{ m^{-1} }[/math] | 1.09846377155094E+07 [math]\displaystyle{ m^{-1} }[/math] | -0.1814% |
| Counterclockwise | 1.09647449477397E+07 [math]\displaystyle{ m^{-1} }[/math] | 1.09636035647508E+07 [math]\displaystyle{ m^{-1} }[/math] | 0.0104% |
And our best estimates of our measured Rydberg Constants:
| Measured Rydberg Constants (in m-1) | ||
|---|---|---|
| Hydrogen | Clockwise | 10979780.2016246 [math]\displaystyle{ \pm }[/math] 2810.24625 |
| Counterclockwise | 10958339.7614656 [math]\displaystyle{ \pm }[/math] 3908.3275 | |
| Deuterium | Clockwise | 10984637.7155094 [math]\displaystyle{ \pm }[/math] 6215.96625 |
| Counterclockwise | 10963603.5647508 [math]\displaystyle{ \pm }[/math] 3312.62625 |
Conclusions and Remarks
I think that there was very little systematic error in this experiment, other than the uncertainty of the direction in which the instrument was calibrated (and we tried to get around that by taking measurements by repeating measurements by turning the knob one way and then the other). There was obviously some random error when measuring the wavelengths of the atomic spectra, since we didn't record the exact same number over and over. By taking five measurements for each atomic emission line, though, I think we were able to control the effects of random error on our final calculations. Because of this, we were able to measure one of the fundamental constants of atomic physics to a high degree of precision. Using an old, clunky spectrometer and getting results within 0.1% of the expected value of the Rydberg Constant for hydrogen-like elements! I think is really quite remarkable. I was very satisfied! I think it also shows the central limit theorem in action, which was kind of exciting.
I would probably do things slightly differently, were I to repeat this experiment. The lab manual neglected to give a procedure for calibrating the instrument, so we spent quite a while fiddling around with all the knobs. Once we finally figured out how to calibrate it, we neglected to record some very important information about the calibration procedure. The spectrometer has a fair amount of backlash and therefore is usually calibrated for taking measurements by turning the knob one way. Unfortunately, we forgot to remember which way the instrument was calibrated, so we just took twice as many measurements - some by turning the knob clockwise only, some by turning the knob counterclockwise only. It's not too hard to do that, but it takes more time.
Lab 3: Planck's Constant
Purpose
The purpose of this lab is to experimentally measure the value of Planck's Constant by measuring the stopping voltage of known wavelengths of light. A mercury emission lamp is used as the source of light. The light is diffracted by a diffraction grating and is focused using a lens onto a vacuum photodiode tube. The light knocks electrons off of a plate in the photodiode tube and they collect on another plate. The amassed electrons on the collector plate create an electric field which will prevent further electrons from reaching the anode (their kinetic energy won't be sufficient to overcome the potential barrier). The potential of the anode, called a stopping potential or stopping voltage, is measured using a digital multimeter. A unity gain op-amp (an op-amp is an operational amplifier) is used to ensure there is a very high resistance for terminals for a voltmeter, in order to prevent electrons from leaving the anode ("current leaking").
Data
The first part of the lab was designed to demonstrate that the stopping voltages are independent of the intensity of light striking the photodiode tube. It did demonstrate this, and we also found that it takes longer for the photodiode tube to build up enough charge to reach the stopping potential for lower intensities of light. These data can be seen here.
The objective of the second part of the lab was to find the ratio of Planck's constant over the charge of the electron by looking at the slope of a linear regression of the stopping voltages vs. frequencies. I had some trouble figuring out how to estimate the error of these data, but ended up with some acceptable answer, I think. You can see the data here.
- My best estimate of Planck's constant based on the data we took was 7.14817E-34 +2.03352E-36/-4.06703E-36.
- (To get this estimate, I took the average of the 1st and 2nd order maxima stopping potentials. The error estimate was found by doing a linear regression on the largest and smallest values of the atomic emission spectral lines' stopping potentials. I don't know whether that was a good way to do it, and it looks a little weird since my error estimate is not symmetric; the deviation to the lower end of the estimate is larger than that to the high end - in fact, it seems to be exactly twice as large! This is rather odd.)
Conclusions and Remarks
I didn't particularly enjoy the lab manual's explanation of photodiode tubes and such. It left some questions in my mind; what was the unity gain op-amp used for? Why did the h/e apparatus have a power supply? I also think if I were to do this lab over, I would take more measurements of the first order maxima stopping voltages. I wasn't very satisfied with my best estimate of the Planck's constant, and I believe that more data points would help. I also think that there is some rather astounding systematic error sources somewhere; the relative error of my best estimate is quite low, but my mean estimate for it is almost 10% off of the accepted value. Perhaps if the lab had been completely dark, it would have produced slightly more accurate results, I'm not sure. I think the digital multimeter may have been slightly broken, as well, since it displayed 0.04 V when the h/e apparatus was shorted out (it should have shown 0.00 V). Or, maybe the unity gain op-amp wasn't entirely precise; I think that the ratio of resistances on op-amp inputs is supposed to decide the gain it has - maybe the resistances weren't precisely the same, but within some tolerance acceptable to the manufacturer.
Lab 4: Speed of Light
SJK 00:48, 25 October 2007 (CDT)

Really great job on this lab! You accepted the challenge of squeezing the best data possible out of less than perfect equipment, and got great results! Very nice job with the data and error analysis.
Purpose
We set out to measure the speed of light using a stopwatch and running really fast. It didn't work too well, so we figured we might revert to the lab manual.SJK 00:30, 25 October 2007 (CDT)

Hey! So you know where the stopwatch is? Anyway, sorry about that today. I spoke with Bill Miller, and he said he's going to look for some this weekend, so hopefully we will be all set next week.
It turns out that the writeup in the lab manual is adequate, if somewhat dense.
In order to measure the speed of light, we didn't build a huge rotating mirror assembly like Michelson did (his work, by the way, is very impressive. His early attempts to measured c produced 299,910±50 km/s - and this was way back in 1879! By 1922, he was able to tweak his methodology to produce 299,796±4 km/s. That's remarkable!) Instead, we used an electronic setup. In our setup, an LED sends a pulse of light to a photomultiplier tube. A device called a Time Amplitude Converter (TAC) is triggered once by a circuit which discharges a capacitor to blink the LED and is triggered once again by the PMT registering a drop in voltage caused by the incident photons. The TAC produces a voltage proportional to the time delay between triggering events. By using an oscilloscope we can measure the voltage at the peak of the pulse the TAC produces. By varying the distance the light has to travel and recording the resulting TAC voltages, we can find the speed of light. Converting the voltages to time (which is simple; just multiply by 5*10-9), plotting the data, time vs. distance, and finding the slope of the best fit line should produce a reasonable estimate for the speed of light.
Data
The slope of these data was found to be [math]\displaystyle{ 3.063 \cdot 10^8 }[/math], with a standard error of [math]\displaystyle{ 1.83 \cdot 10^7 }[/math], making the best estimate of the speed of light [math]\displaystyle{ (3.063 \pm 0.18) \cdot 10^8 \frac{m}{s} }[/math].
This is a relative error of 5.96%, and the value 3.063E8 m/s is 2.17% different from the accepted value of the speed of light, [math]\displaystyle{ 2.9979 \cdot 10^8 \frac{m}{s} }[/math] (according to Wikipedia).
Conclusions and Remarks
The use of a multichannel analyzer, as suggested by Tomas Mondragon, might smooth things up a bit.
Also, there appears to be an outlier in the plot of our data; the time measured for a distance of 140 cm into the tube was less than the time measured for a distance of 130 cm into the tube. I don't know what went wrong here, but it's worth noting. The TAC must have a delay of at least 2 nanoseconds between triggering events; perhaps by positioning the LED module 140 cm into the tube we made the "halt" event trigger before the "start" event (as the wires are of unequal length; the wire between the LED module and TAC is much, much longer than the wire between the PMT and TAC). We were supposed to use a time delay mechanism to add a time delay to the "halt" trigger, but we found it to be unnecessary for longer distances between the LED and PMT.
Since the readings on the DSO were unstable, with the minimum voltage of the PMT fluctuating significantly, I'm pretty happy with these results.
Lab 5: Millikan Oil Drop
Purpose
Using PASCO Scientific's Millikan Oil Drop Apparatus (Model AP-8210), experimentally determine the value of the elementary charge and confirm the quantization of charge.
By spraying mineral oil of known density into the apparatus with an atomizer and measuring the time it takes a droplet to fall one major reticle line on the viewing scope (which is 0.5 mm), we can extrapolate the radius (and therefore volume and mass) of the droplet using Stoke's Law (which describes the behavior of spherical objects in a fluid). By applying voltage to the two plates above and below the viewing chamber, we subject the droplet to an electric field. If there is a net charge on our droplet, the electric field will exert a force on the droplet and make it rise or fall, depending on the polarity. In the spirit of Giordi LaForge, we might have to reverse the polarity (and that obviously fixes any problem which might arise on the Enterprise) in order for the droplet to rise. Measuring the time it takes the droplet to rise one major reticle line and doing some algebra will approximate the net charge of the droplet.
Data and Analysis
My lab partner (Kyle Martin) and I were able to record the fall times and rise times for 18 oil droplets. We were able to measure these times more than once on 11 of these droplets. Following the equations laid out in the PASCO lab manual, we were able to determine the charges of these 18 droplets. It's reasonable to assume that the charge on any given droplet is constant, and therefore we averaged the calculated charges for those droplets we were able to measure several times. Looking at the calculated charges, it seems that the charges are grouped. Taking the mean of the smallest group to be the elementary charge (which seems reasonable to me, though we took data from only 18 droplets and therefore could be missing charge of smaller magnitude; I mean, we haven't taken nearly enough data to determine for certain that the smallest charge that we see is in fact the elementary charge. If we were to get serious about this, it might be necessary to take a quintillion measurements to rule out fractional charge.)
Anyway, it's assumed that the mean of the first group is the elementary charge and each subsequent group is an integer multiple of the elementary charge. While this isn't a rigorous proof of the quantization of charge, Millikan had the notion of quantized charge before commencing his measurements also - see J.J. Thompson's "corpuscles" of charge.
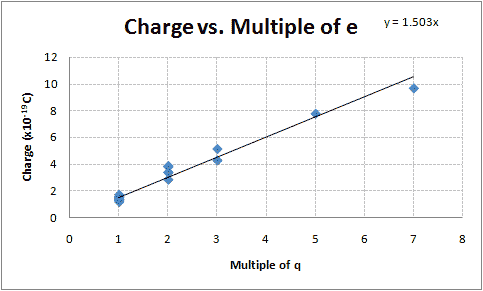
| Average Charge Per Drop | Group |
|---|---|
| 1.170284691 | Group 1 |
| 1.261815586 | Group 1 |
| 1.326204984 | Group 1 |
| 1.357593958 | Group 1 |
| 1.384181124 | Group 1 |
| 1.495305796 | Group 1 |
| 1.499256751 | Group 1 |
| 1.579841893 | Group 1 |
| 1.705486127 | Group 1 |
| 2.834670357 | Group 2 |
| 3.377621558 | Group 2 |
| 3.379771496 | Group 2 |
| 3.788927915 | Group 2 |
| 3.858074883 | Group 2 |
| 4.273635645 | Group 3 |
| 5.132206708 | Group 3 |
| 7.765736898 | Group 4 |
| 9.667025415 | Group 5 |
| Average (x10^-19 C) | Suspected Multiple of q | |
|---|---|---|
| Group 1 | 1.419996768 | 1 |
| Group 2 | 3.447813242 | 2 |
| Group 3 | 4.702921176 | 3 |
| Group 4 | 7.765736898 | 5 |
| Group 5 | 9.667025415 | 7 |
Conclusions and Remarks
Graphing the calculated charges vs. their suspected integer multiple of e-, there appears to be a linear relationship (See Figure 1). Using Excel to determine the slope of the line that fits this data (using the least squares method) and its uncertainty produces my best estimate of the value of the elementary charge:
[math]\displaystyle{ (1.50 \pm 0.039)\times 10^{-19}\;C }[/math]
This value is 6.1% less than the accepted value of [math]\displaystyle{ 1.602\times 10^{-19}\;C }[/math].
Possible sources of error include Brownian motion (as the droplets of oil are very small), random errors associated with timing (delay between the droplet crossing the major reticle and the stopwatch button being pressed, etc.), losing track of the oil droplet through the viewing scope, uncertainties of the electric field, uncertainties of the temperature (unsteady resistance readings of the thermistor, or problems interpreting the resistance as a temperature), inaccuracies in the determination of the viscosity of air (the table is for dry air, what will the effect of humidity be?), uncertainties of the barometric pressure, inaccuracies of Stokes' Law (or the assumptions he made to derive the equation) and uncertainties of the density of our mineral oil.
Were I to repeat this experiment, I would have tried very hard to find a microscope camera or to use a projector of some sort to observe the viewing chamber, as looking through the viewing scope for extended periods of time is very wearisome. I would also want to take many more measurements of the fall times and rise times, as 18 droplets are inadequate for determining the minimum quantum of charge with any certainty. The apparatus also has a radiation source which is designed to knock off electrons from the droplets, allowing subsequent measurements of the same droplet to be examining less net charge. We weren't able to use this feature of the apparatus, as we had trouble following a single droplet for many up-and-down cycles.
Also, reading the PASCO lab manual, I found that Millikan used a small cell of water between the lamp and viewing chamber to absorb the heat produced by the lamp and effectively thermally isolate the viewing chamber from the lamp. Our apparatus didn't have this feature, and it's likely that as our experiment progressed the temperature of our viewing chamber increased. My lab partner and I didn't record the resistance of the thermistor for each trial we ran, as we perhaps should have.
Formal Report
I did my formal report on the Speed of Light lab. I chose to typeset it in LaTeX, and it output a very pretty PDF which you should definitely read.
Koch comments on rough draft
Jesse, Here are my comments on your rough draft. I'll send an email with grade estimate. Additional:
- Calibration! Add a discussion as to how the TAC was calibrated (and also do this if you did not previously). How do we know scope is working properly, etc.
Links to Lab Entries
| Wednesday Labs | ||||
|---|---|---|---|---|