SBB09Ntbk-Simina Ticau
upaG Autotransporter short version
Hydroxyapatite-binding peptide
Magnetic Bead Binding Peptide
*Simina Ticau 00:57, 7 March 2009 (EST):
- got the sequence analysis for what I thought was clone 1 of M10027: perfect match to M10043
- first clone of upaG (M10026) : there was a 1bp deletion in the BamHI site
Simina Ticau 14:31, 4 March 2009 (EST):
- analyzed the sequencing that came in: I mixed up the tubes and both clones of Magnetic Bead BP got sequenced (none of the Hydroxyapatite BP) - both clones for Magnetic Bead BP looked perfect (sbb008 and sbb010); filled in all the wiki sites/google docs for that
for upaG:
- plate looked good; had many colonies
MINIPREP PURIFICATION OF DNA
1. Pellet 1.5 mL saturated culture by spinning full speed, 30 seconds. 2. Dump supernatant, repeat to pellet another 1.5 mL (for a total of 3 mL) 3. Add 250uL of P1 buffer into each tube. Resuspend the cells using a vortexer. 4. Add 250uL of P2 buffer (a base that denatures everything and causes cells to lyse). Gently mix up and down. Solution should become clearer. 5. Add 350uL of N3 buffer (an acid of pH ~5 that causes cell junk - including protein and chromosomal DNA - to precipitate, and leaves plasmids and other small molecules in solution). Slowly invert a few times, then shake. 6. Spin in centrifuge at top speed for 5 minutes. 7. Label blue columns with an alcohol-resistant lab pen. 8. Pour liquid into columns, and place the columns into the centrifuge. Spin at 12000 rpm for 30 seconds. 9. Dump liquid out of the collectors under the columns (the DNA should be stuck to the white resin) 10. Wash each column with 500 uL of PB buffer. 11. Spin in centrifuge at 12000rpm for approximately 15 seconds, then flick out the liquid again. 12. Wash with 750uL of PE buffer (washes the salts off the resins). 13. Spin in centrifuge at 12000rpm for approximately 15 seconds and flick out liquid again. 14. Spin in centrifuge at full speed for 1 minute to dry off all water and ethanol. 15. Label new tubes and put columns in them. 16. Elute them by squirting 50uL of water down the middle of the column (don't let it stick to the sides). 17. Spin in centrifuge at top speed for 30 seconds. 18. Take out columns and cap the tubes. 19. Clean up - note the P1 buffer is stored at 4degC and all the rest at room temperature.
RESTRICTION MAPPING
3uL of miniprep DNA 5ul H20 1uL of NEB Buffer 2 0.5uL EcoRI 0.5uL BamHI *Incubate at 37 degrees on the thermocycler for 1hr *run out on a gel to check size (should be 2974bp, 282bp)
- sent it in for sequencing: well G2 on both plates, sbb021/022
Simina Ticau 14:32, 2 March 2009 (EST):
for Hydroxyapatite BP and Magnetic Bead BP:
RESTRICTION MAPPING
3uL of miniprep DNA 5ul H20 1uL of NEB Buffer 2http://openwetware.org/index.php?title=SBB09Ntbk-Simina_Ticau&action=edit# 0.5uL EcoRI 0.5uL XhoI *Incubate at 37 degrees on the thermocycler for 1hr *run out on a gel to check size Hydroxyapatite should be 2026bp, 1196bp Magnetic Bead BP should be 2011bp, 1196bp

lanes 1-2: Hydroxyapatite BP
lanes 3-4: Magnetic Bead BP
- sent them in for sequencing; filled in all the forms
for upaG:
LIGATION
*Set up the following reaction: 6.5uL ddH2O 1uL T4 DNA Ligase Buffer (small red or black-striped tubes) 1uL pBca9495KC-Bca1144#5 1uL Insert digest 0.5uL T4 DNA Ligase *Pound upside down on the bench to mix *Give it a quick spin to send it back to the bottom of the tube *Incubate on the benchtop for 30min *Put on ice and proceed to the transformation
TRANSFORMATION
Competent cells are stored as 200uL aliquots in the -80 freezer as a communal stock 1. Thaw a 200 uL aliquot of cells on ice 2. Add 50 uL of water 3. Add 30 uL of KCM salts 4. Put your ligation mixture on ice, let it cool a minute or two 5. Add 75 uL of the cell cocktail to the ligation, pipette up and down gently to mix 6. Let sit on ice for 10 min 7. Heat shock for 2 min at 42 8. Put back on ice for 1 min 9. For ampicillin selection, you can plate immediately, otherwise: 10. Add 100uL of LB, let shake in the 37 degree incubator for 40 min 11. Plate on selective antibiotics (KC), let incubate overnight
Simina Ticau 14:10, 27 February 2009 (EST)
for Hydroxyapatite BP and Magnetic Bead BP:
- plats looked good; both had many colonies
MINIPREP PURIFICATION OF DNA
1. Pellet 1.5 mL saturated culture by spinning full speed, 30 seconds. 2. Dump supernatant, repeat to pellet another 1.5 mL (for a total of 3 mL) 3. Add 250uL of P1 buffer into each tube. Resuspend the cells using a vortexer. 4. Add 250uL of P2 buffer (a base that denatures everything and causes cells to lyse). Gently mix up and down. Solution should become clearer. 5. Add 350uL of N3 buffer (an acid of pH ~5 that causes cell junk - including protein and chromosomal DNA - to precipitate, and leaves plasmids and other small molecules in solution). Slowly invert a few times, then shake. 6. Spin in centrifuge at top speed for 5 minutes. 7. Label blue columns with an alcohol-resistant lab pen. 8. Pour liquid into columns, and place the columns into the centrifuge. Spin at 12000 rpm for 30 seconds. 9. Dump liquid out of the collectors under the columns (the DNA should be stuck to the white resin) 10. Wash each column with 500 uL of PB buffer. 11. Spin in centrifuge at 12000rpm for approximately 15 seconds, then flick out the liquid again. 12. Wash with 750uL of PE buffer (washes the salts off the resins). 13. Spin in centrifuge at 12000rpm for approximately 15 seconds and flick out liquid again. 14. Spin in centrifuge at full speed for 1 minute to dry off all water and ethanol. 15. Label new tubes and put columns in them. 16. Elute them by squirting 50uL of water down the middle of the column (don't let it stick to the sides). 17. Spin in centrifuge at top speed for 30 seconds. 18. Take out columns and cap the tubes. 19. Clean up - note the P1 buffer is stored at 4degC and all the rest at room temperature.
Simina Ticau 14:53, 25 February 2009 (EST)
for Hydroxyapatite BP and Magnetic Bead BP:
LIGATION
*Set up the following reaction: 6.5uL ddH2O 1uL T4 DNA Ligase Buffer (small red or black-striped tubes) 1uL pBca9495AK-Bca1144 1uL Insert digest 0.5uL T4 DNA Ligase *Pound upside down on the bench to mix *Give it a quick spin to send it back to the bottom of the tube *Incubate on the benchtop for 30min *Put on ice and proceed to the transformation
TRANSFORMATION
Competent cells are stored as 200uL aliquots in the -80 freezer as a communal stock 1. Thaw a 200 uL aliquot of cells on ice 2. Add 50 uL of water 3. Add 30 uL of KCM salts 4. Put your ligation mixture on ice, let it cool a minute or two 5. Add 75 uL of the cell cocktail to the ligation, pipette up and down gently to mix 6. Let sit on ice for 10 min 7. Heat shock for 2 min at 42 8. Put back on ice for 1 min 9. For ampicillin selection, you can plate immediately, otherwise: 10. Add 100uL of LB, let shake in the 37 degree incubator for 40 min 11. Plate on selective antibiotics, let incubate overnight
for upaG:
DIGEST
*Set up the following reaction: 8uL of eluted PCR product 1uL of NEB Buffer 2 0.5uL EcoRI 0.5uL BamHI *Incubate at 37 degrees on the thermocycler for 1hr
REGULAR ZYMO CLEANUP
The following procedure removes the polymerase, dNTPs, buffer, and most of the oligonucleotides from a PCR reaction. It also will remove the buffer and restriction enzymes from a restriction digest reaction. 1. Add 180 uL of Zymo ADB buffer (brown bottle) to a 33uL or 50uL reaction (min. is 3 volumes) 2. Transfer into the Zymo column (small clear guys) 3. spin through (15 secs at max speed, ie around 15,000 rpm), discard waste. 4. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 5. spin through, discard waste. 6. Add 200 uL of PE or Zymo Wash buffer 7. spin through, discard waste. 8. spin for 90 seconds, full speed to dry. 9. elute with water into a fresh Eppendorf tube, use the same volume of water as the volume of the original reaction (used 10ul)
Simina Ticau 14:48, 23 February 2009 (EST)
for upaG:
GEL:
- 5ul sample + 1ul dye - ladder: 3-5ul - run at 100V for about 20 minutes
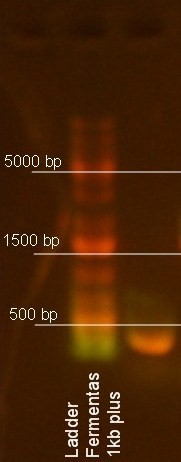
(used David's edited pic and cropped it)
upaG 300bp
REGULAR ZYMO CLEANUP:
The following procedure removes the polymerase, dNTPs, buffer, and most of the oligonucleotides from a PCR reaction. It also will remove the buffer and restriction enzymes from a restriction digest reaction. 1. Add 180 uL of Zymo ADB buffer (brown bottle) to a 33uL or 50uL reaction (min. is 3 volumes) 2. Transfer into the Zymo column (small clear guys) 3. spin through (15 secs at max speed, ie around 15,000 rpm), discard waste. 4. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 5. spin through, discard waste. 6. Add 200 uL of PE or Zymo Wash buffer 7. spin through, discard waste. 8. spin for 90 seconds, full speed to dry. 9. elute with water into a fresh Eppendorf tube, use the same volume of water as the volume of the original reaction (used 33ul)
for HydroxyapatiteBP and Magnetic Bead BP:
SMALL-FRAG ZYMO CLEANUP:
The following procedure removes the polymerase, dNTPs, buffer, and most of the oligonucleotides from a PCR reaction for fragments smaller than 300bp. It also will remove the buffer and restriction enzymes from a restriction digest reaction. 1. Add 100 uL of Zymo ADB buffer (brown bottle) to the reaction. 2. Transfer into the Zymo column (small clear guys) 3. Add 500uL of Ethanol and pipette up and down to mix 4. spin through, discard waste. 5. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 6. spin through, discard waste. 7. Add 200 uL of PE or Zymo Wash buffer 8. spin through, discard waste. 9. spin for 90 seconds, full speed to dry. 10. elute with water (50ul) into a fresh Eppendorf tube
DIGEST:
*Set up the following reaction: 50uL of eluted PCR product 5.7uL of NEB Buffer 2 1uL EcoRI 1uL BamHI *Incubate at 37 degrees on the thermocycler for 1hr
ANOTHER SMALL FRAG ZYMO CLEAN-UP
- elute in 50ul
Simina Ticau 14:12, 18 February 2009 (EST)
Got the oligos: no longer working on the Beta Helix Repeats basic part, so I'm no longer going to be using Ost005F and Ost 006R
Ost001F 25.70nmol Tm = 61.1
Ost002R 26.30nmol Tm = 66.9
Ost003F 26.30nmol Tm = 69.0
Ost004R 29.70nmol Tm = 70.6
Ost007F 27.40nmol Tm = 64.5
Ost008R 31.40nmol Tm = 66.0
Today I set up the Wobble reactions for Hydroxyapatite-binding peptide and Magnetic Bead Binding Peptide and the PCR reaction for upaG Autotransporter short version.
Left them in the respective machines
Updated the pages for each of the projects
For Monday: - start gel for the PCR product - cleanup for the Wobble reactions - clean-up for the PCR
Simina Ticau 13:19, 11 February 2009 (EST)
Today we went through more protocols; I wrote down my notes here so I would have everything on one page for Monday.
OLIGOS :
- they will be at a concentration of 28.80 nmol (may be at a diff concentration, need to check) and we want a final concentration of 100uM --> add 288 ul of water (if 28.80 nmol) (make sure you spin down the tubes before adding the water to make sure everything's at the bottom); mix well (tube rack) and spin down again --> oligo concentrations are now 100uM
PCR :
- first need to make an oligo dilution of: 9uL Water 1uL 100uM oligo You can throw away the remainder of the diluted oligo when you are done, but hold onto your stock tube! Set up the following reaction in a PCR tube (this is only for regular PCR, not Wobble Reaction): 24uL ddH2O 3.3uL 10x Expand Buffer "2" 3.3uL dNTPs (2mM in each) 1uL Oligo 1, 10uM 1uL Oligo 2, 10uM 0.5uL Expand polymerase "1" 0.5uL Template DNA
There are different programs: have to choose between C55, C2K55, C4K55, C8K55
If you get no product or poor yield of product, repeat the same PCR reaction as two different variants, both with the “45” program. The first reaction will be the same composition as the first. For the second one, don’t add 3.3 uL of the water and instead use the volume for 3.3 uL of DMSO (dimethylsulfoxide). If these PCRs fail as well, well you'd either redesign, use a different template source, or give up.
GEL:
- 5ul sample + 1ul dye - ladder: 3-5ul - run at 100V for about 20 minutes
REGULAR ZYMO CLEANUP:
The following procedure removes the polymerase, dNTPs, buffer, and most of the oligonucleotides from a PCR reaction. It also will remove the buffer and restriction enzymes from a restriction digest reaction. 1. Add 180 uL of Zymo ADB buffer (brown bottle) to a 33uL or 50uL reaction (min. is 3 volumes) 2. Transfer into the Zymo column (small clear guys) 3. spin through (15 secs at max speed, ie around 15,000 rpm), discard waste. 4. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 5. spin through, discard waste. 6. Add 200 uL of PE or Zymo Wash buffer 7. spin through, discard waste. 8. spin for 90 seconds, full speed to dry. 9. elute with water into a fresh Eppendorf tube, use the same volume of water as the volume of the original reaction
DIGEST:
For PCR products, you will only digest a portion of your purified PCR product. Note that you must make a minor modification to the procedure if your DNA is shorter than 300bp (you add a little isopropanol to the ADB after melting) *Set up the following reaction: 8uL of eluted PCR product 1uL of NEB Buffer 2 0.5uL EcoRI 0.5uL BamHI *Incubate at 37 degrees on the thermocycler for 1hr *Run an agarose gel, and melt with 600uL ADB buffer at 55 degrees. ****NOTE: If you are running short of time, this is an acceptable stopping point *If the DNA is shorter than 300bp, add 250uL of isopropanol and mix prior to loading it on the column
Simina Ticau 14:52, 9 February 2009 (EST)
- got the 2nd project ball
- made the construction files for Magnetic Bead BP & Beta Helix Repeats
- updated oligo page/construction file page/excel spreadsheet
Construction of Beta Helix Repeats basic part
PCR Ost005F and Ost006R on E. coli avian APEC genomic DNA (703bp, EcoRI/BamHI)
Sub into JCA_pBca9495AK-Bca1144 (EcoRI/BamHI, 3171+910, L)
Product is JCA_pBca9495AK-M10042 {Beta Helix Repeats}
----------------------------
Ost005F Forward oligo for Beta Helix Repeats
ccaaaGAATTCatgAGATCTagtgtcttcaacggcaccg
Ost006R Reverse oligo for Beta Helix Repeats
ctagcGGATCCtgtacgcatgacaaatgctgac
Wobble Ost007F/Ost008R (52 bp, EcoRI/BamHI)
Sub into JCA_pBca9495AK-Bca1144 (EcoRI/BamHI, 3171+910, L)
Product is JCA_pBca9495AK-M10043 {Magnetic Bead BP}
----
Ost007F Forward construction of Magnetic Bead BP basic part
ccaaaGAATTCatgAGATCTCACCACAAATACTGGCACCG
Ost008R Reverse construction of Magnetic Bead BP basic part
ctagcGGATCCACGGTGCCAGTATTTGTGGTG
to do: Read articles
Simina Ticau 14:20, 4 February 2009 (EST)
- got the project ball
- started working on the wiki & looking at the articles
- made the construction file for upaG & HydroxyapatiteBP
- updated oligo page/construction file page/excel spreadsheet
Construction of upaG basic part
PCR Ost001F and Ost002R on CFT073 genomic DNA (298bp (282+16), EcoRI/BamHI)
Sub into JCA_pBca9495KC-Bca1144 (EcoRI/BamHI, 2974+910, L)
Product is JCA_pBca9495KC-M10026 {upaG}
----------------------------
Ost001F Cloning of upaG ccaaaGAATTCatgAGATCTgttgagatggataacaaactg
Ost002R Cloning of upaG ctagcGGATCCttaCCACtgaataccggcaccgag
Wobble Ost003F/Ost004R (67 bp, EcoRI/BamHI)
Sub into JCA_pBca9495AK-Bca1144 (EcoRI/BamHI, 3171+910, L)
Product is JCA_pBca9495AK-M10027 {HydroxyapatiteBP}
----
Ost003F Forward construction of HydroxyapatiteBP basic part
ccaaaGAATTCatgAGATCTgcgccgtggcatctgagcagccagtatag
Ost004R Reverse construction of HydroxyapatiteBP basic part
ctagcGGATCCggtgcggctatactggctgctcagatgc
to do: Read articles