SBB10Ntbk-AndrewSaarni: Difference between revisions
No edit summary |
No edit summary |
||
| Line 416: | Line 416: | ||
'''(*COMPLETE*)''' sbb12 progress: '''Design''' --> '''Assembly''' --> '''Amplification''' --> '''Zymo Clean-up''' --> '''Restriction Digest''' --> '''Preparative Gel''' --> '''Zymo Gel Purification''' --> '''Transformation''' --> '''Miniprep''' --> '''Mapping''' --> '''Sequencing''' <br> | '''(*COMPLETE*)''' sbb12 progress: '''Design''' --> '''Assembly''' --> '''Amplification''' --> '''Zymo Clean-up''' --> '''Restriction Digest''' --> '''Preparative Gel''' --> '''Zymo Gel Purification''' --> '''Transformation''' --> '''Miniprep''' --> '''Mapping''' --> '''Sequencing''' <br> | ||
sbb14 progress: '''Initial PCR Reactions''' --> '''Preparative Gel''' --> '''Zymo Gel Purification''' --> '''Secondary PCR Reactions''' --> '''Analytical Gel''' --> '''Secondary PCR Reactions (initial set failed)''' --> '''Zymo Cleanup''' --> '''Restriction Digest''' --> '''Preparative Gel''' --> '''Zymo Gel Purification''' --> '''Transformation''' --> Miniprep --> Mapping --> Sequencing<br> | sbb14 progress: '''Initial PCR Reactions''' --> '''Preparative Gel''' --> '''Zymo Gel Purification''' --> '''Secondary PCR Reactions''' --> '''Analytical Gel''' --> '''Secondary PCR Reactions (initial set failed)''' --> '''Zymo Cleanup''' --> '''Restriction Digest''' --> '''Preparative Gel''' --> '''Zymo Gel Purification''' --> '''Transformation''' --> Miniprep --> Mapping --> Sequencing<br> | ||
===sbb12=== | ===sbb12 (*COMPLETE*)=== | ||
The sequencing data for clone 1 was perfect except for a base insertion in the BglII site (agaTtct).<br> | The sequencing data for clone 1 was perfect except for a base insertion in the BglII site (agaTtct).<br> | ||
Clone 2 was perfect aside from a point mutation in the EcoR1 site (gGattc).<br> | Clone 2 was perfect aside from a point mutation in the EcoR1 site (gGattc).<br> | ||
Revision as of 16:01, 10 March 2010
Design
Construction files for two parts (Sleeping Beauty 3'TR and PhiC31 Integrase with RBS)
sbb12
The sbb12 part is small enough to be entirely synthesized using PCA. The GeneDesign program [1]
was used to generate appropriate oligos for the synthesis.
1) sbb12: Sleeping Beauty 3'TR ---------------------------------------------------------- Pool AS001-F through AS008-R, assemble by PCA PCR AS001-F/AS008-R on PCA reaction (259 bp, EcoRI/BamHI) Substitute in pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L) Product is pBjk2741-sbb12 ---------------------------------------------------------- AS001-F PCA Assembly of sbb12 CCATAGAATTCATGAGATCTTTGAGTGTATGTTAACTTCTGACCCACTGG AS002-R PCA Assembly of sbb12 ATTTCAGCTTTTATTTCTTTCATCACATTCCCAGTGGGTCAGAAGTTAAC AS003-F PCA Assembly of sbb12 AAAGAAATAAAAGCTGAAATGAATCATTCTCTCTACTATTATTCTGATAT AS004-R PCA Assembly of sbb12 AGGATCACCACTTTATTTTAAGAATGTGAAATATCAGAATAATAGTAGAG AS005-F PCA Assembly of sbb12 TAAAATAAAGTGGTGATCCTAACTGACCTTAAGACAGGGAATCTTTACTC AS006-R PCA Assembly of sbb12 CACTTTTTCACAATTCCTGACATTTAATCCGAGTAAAGATTCCCTGTCTT AS007-F PCA Assembly of sbb12 TCAGGAATTGTGAAAAAGTGAGTTTAATGTATTTGGCTAAGGTGTATGTA AS008-R PCA Assembly of sbb12 CTGATGGATCCCAGTTGAAGTCGGAAGTTTACATACACCTTAGCCAAAT
sbb14
For construction, sbb14 will be created as three sections which will then be connected by SOEing PCR.
Each section is constructed by amplifying a template DNA from a plasmid using standard PCR amplification.
The resulting sections have complementary overlapping sequences (either at the beginning, end, or both),
which allow the sections to be attached to each other by SOEing PCR to produce the final part.
Additionally, a strong RBS was generated using [2] and added to the first
forward oligo to be incorporated into the first section.
sbb14: PhiC31 Integrase
----------------------------------------------------------
PCR AS009-F/AS010-R on Bca1623 6-9 (976bp, gp=A)
PCR AS011-F/CA1674 on Bca1559 7-7 (514bp, gp=B)
PCR CA1675/AS012-R on Bca1559 7-1 (444bp, gp=C)
----------------------------------------------------------
PCR AS009-F/AS012-R on A+B+C (1868 bp, EcoRI/BamHI)
Sub in pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-sbb14 {rbs.phiC31>}
----------------------------------------------------------
AS009-F Forward PCR of Part 1 of PhiC31 with RBS ccataGAATTCatgAGATCTCAGATCATATATAAGGAGGTACATatgGACACGTACGCGGGTGCTTACG
AS010-R SOEing of Part 1 of PhiC31 cggtaaccctcaatcttcgtGGTCGGCGTGCCGTCCGGCTTCTT
AS011-F SOEing of Part 2 of PhiC31 AAGAAGCCGGACGGCACGCCGACCacgaagattgagggttaccg
CA1674 PCA assembly of phiCthreeprime (Bca1659) GGGCGTCGGCGCGCTCCGCAACAAGGTTCGCCCGTTCGCCGCTCTTCTCAG
CA1675 PCA assembly of phiCthreeprime (Bca1659) TGCGGAGCGCGCCGACGCCCTGAACGCCCTTGAAGAGCTGTACGAAGACCG
AS012-R Reverse PCR of Part 2 of PhiC31 atcagGGATCCCGCCGCTACGTCTTCCGT
Part: GATCTCAGATCATATATAAGGAGGTACATatgGACACGTACGCGGGTGCTTACGACCGTCAGTCGCGCGAGCGCGAAAATTCGAGCGCAGCAAGCCCAGCGACACAGCGTAGCGCCAACGAAGACAAGGCGGCCGACCTCCAGCGCGAAGTCGAGCGCGACGGGGGCCGGTTCAGGTTCGTCGGGCATTTCAGCGAAGCGCCGGGCACGTCGGCGTTCGGGACGGCGGAGCGCCCGGAGTTCGAACGCATCCTGAACGAATGCCGCGCCGGGCGGCTCAACATGATCATTGTCTATGACGTGTCGCGCTTCTCGCGCCTCAAGGTCATGGACGCGATTCCGATTGTCTCGGAATTGCTCGCCCTGGGCGTGACGATTGTTTCCACTCAGGAAGGCGTCTTCCGGCAGGGAAACGTCATGGACCTGATTCACCTGATTATGCGGCTCGACGCGTCGCACAAAGAATCTTCGCTCAAGTCGGCGAAGATTCTCGACACGAAGAACCTCCAGCGCGAATTGGGCGGGTACGTCGGCGGGAAGGCGCCTTACGGCTTCGAGCTTGTTTCGGAGACGAAGGAGATCACGCGCAACGGCCGAATGGTCAATGTCGTCATCAACAAGCTTGCGCACTCGACCACTCCCCTTACCGGACCCTTCGAGTTCGAGCCCGACGTAATCCGGTGGTGGTGGCGTGAGATCAAGACGCACAAACACCTTCCCTTCAAGCCGGGCAGTCAAGCCGCCATTCACCCGGGCAGCATCACGGGGCTTTGTAAGCGCATGGACGCTGACGCCGTGCCGACCCGGGGCGAGACGATTGGGAAGAAGACCGCTTCAAGCGCCTGGGACCCGGCAACCGTTATGCGAATCCTTCGGGACCCGCGTATTGCGGGCTTCGCCGCTGAGGTGATCTACAAGAAGAAGCCGGACGGCACGCCGACCacgaagattgagggttaccgCATTCAGCGCGACCCGATCACGCTCCGGCCGGTCGAGCTTGATTGCGGACCGATCATCGAGCCCGCTGAGTGGTATGAGCTCCAGGCGTGGTTGGACGGCAGGGGGCGCGGCAAGGGGCTTTCCCGGGGGCAAGCCATTCTGTCCGCCATGGACAAGCTGTACTGCGAGTGTGGCGCCGTCATGACTTCGAAGCGCGGGGAAGAATCGATCAAGGACTCTTACCGCTGCCGTCGCCGGAAGGTGGTCGACCCGTCCGCACCTGGGCAGCACGAAGGCACGTGCAACGTCAGCATGGCGGCACTCGACAAGTTCGTTGCGGAACGCATCTTCAACAAGATCAGGCACGCCGAAGGCGACGAAGAGACGTTGGCGCTTCTGTGGGAAGCCGCCCGACGCTTCGGCAAGCTCACTGAGGCGCCTGAGAAGAGCGGCGAACGGGCGAACCTTGTTGCGGAGCGCGCCGACGCCCTGAACGCCCTTGAAGAGCTGTACGAAGACCGCGCGGCAGGCGCGTACGACGGACCCGTTGGCAGGAAGCACTTCCGGAAGCAACAGGCAGCGCTGACGCTCCGGCAGCAAGGGGCGGAAGAGCGGCTTGCCGAACTTGAAGCCGCCGAAGCCCCGAAGCTTCCCCTTGACCAATGGTTCCCCGAAGACGCCGACGCTGACCCGACCGGCCCTAAGTCGTGGTGGGGGCGCGCGTCAGTAGACGACAAGCGCGTGTTCGTCGGGCTCTTCGTAGACAAGATCGTTGTCACGAAGTCGACTACGGGCAGGGGGCAGGGAACGCCCATCGAGAAGCGCGCTTCGATCACGTGGGCGAAGCCGCCGACCGACGACGACGAAGACGACGCCCAGGACGGCACGGAAGACGTAGCGGCGG
Assembly
2.17.2010
Overall Progress
Performed initial step of PCA assembly of sbb12 and initial step of SOEing PCR for sbb14.
sbb12 progress: Design --> Assembly --> Amplification --> Reassembly --> Cloning
sbb14 progress: Initial PCR Reactions --> Zymo Clean-up --> Secondary PCR Reactions --> Cloning
sbb12
Experimental
Oligo Mixture was provided at 100uM
Initial assembly of the PCA oligo mixture was performed according to the following protocol:
1. 38 uL ddH2O 2. 5 ul 10x expand buffer 3. 5 ul 2mM dNTPs 4. 1 ul oligo mixture (100uM total, mixture of oligos after combination of 100uM stocks) 5. 0.75 ul Expand polymerase
The resulting mixture was then thermo-cycled according to the following program:
1. 2 min initial denature at 94oC 2. 30 sec denature at 94oC 3. 30 sec anneal at 55oC [This should be the overlap temp of your oligos - vary as needed] 4. 30 sec extension at 72oC 5. repeat 2-4 30 times total
Note: thermo-cycling performed by GSI's
sbb14
Experimental
All oligo samples were first diluted to 100uM by adding sufficient water (ddH2O)
Then, 10uM samples of each oligo were prepared by combining 1uL of 100uM Oligo and 9uL of ddH2O
Three PCR tubes were labeled 1,2 and 3.
The following PCR protocol was used:
24uL ddH2O 3.3uL 10x Expand Buffer "2" 3.3uL dNTPs (2mM in each) 1uL Oligo 1, 10uM 1uL Oligo 2, 10uM 0.5uL Expand polymerase "1" 0.5uL Template DNA
A forward and reverse oligo were added to each tube according to the PCR protocol.
Tube 1: AS009-F and AS010-R
Tube 2: AS011-F and CA1674
Tube 3: CA1675 and AS012-R
Tubes 1,2 and 3 were then thermo-cycled according to the 2K55 program because each PCR product
is less than 2000bp.
2.22.2010
Overall Progress
Ran preparative gel for sbb14 SOEing reactions and performed a small-frag Zymo Cleanup followed by Fuzion amplification for sbb12 PCA
sbb12 progress: Design --> Assembly --> Amplification --> Reassembly --> Cloning
sbb14 progress: Initial PCR Reactions --> Preparative Gel --> Zymo Clean-up --> Secondary PCR Reactions
sbb12
Experimental
Initial PCA Assembly reaction was purified by small fragment Zymo cleanup according to the following protocol:
Small-Frag Zymo Cleanup The following procedure removes the polymerase, dNTPs, buffer, and most of the oligonucleotides from a PCR reaction for fragments smaller than 300bp. It also will remove the buffer and restriction enzymes from a restriction digest reaction. 1. Add 100 uL of Zymo ADB buffer (brown bottle) to the reaction. 2. Transfer into the Zymo column (small clear guys) 3. Add 500uL of Ethanol and pipette up and down to mix 4. spin through, discard waste. 5. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 6. spin through, discard waste. 7. Add 200 uL of PE or Zymo Wash buffer 8. spin through, discard waste. 9. spin for 90 seconds, full speed to dry. 10. elute with water into a fresh Eppendorf tube
The purified product was used as the template for the following PCR amplification protocol:
Amplification Now, you need to do an amplification of the correct full-length chunks. Clean up the assembly reaction with a zymo column; don't bother running it on a gel - it'll be a smeary mess and won't really help you. Save the purified product in case this step fails! For the amplification reaction, do a normal phusion program with 1 uL of the cleaned up assembly reaction as template, and using the outermost oligos for the chunk. That is: Recipe 1. 1 uL each outer oligo (10 uM) (Provided as F/R Oligos) 2. .5 uL phusion 3. 10 uL 5x phusion buffer 4. 5 uL 2mM dNTPs 5. 32.5 uL H2O 6. 1 uL Template Program 1. 2 min initial denature at 94oC 2. 30 sec denature at 94oC 3. 30 sec anneal at 60oC [This should be high, as your outer oligos now have a huge overlap with the correct product] 4. 30 sec extension at 68oC 5. repeat 2-4 30 times total
Tube labeled AS Fuzion 04
sbb14
Experimental
6uL of each PCR reaction and 4uL of loading buffer were added to 3 Eppendorf tubes, labeled AS01, AS02 and AS03.
Resulting mixtures were then run on preparative gels.
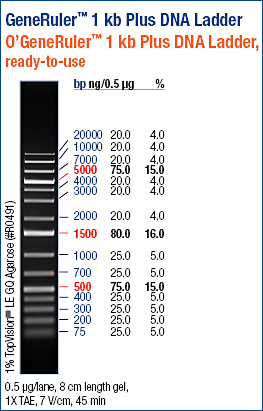

Lane 3 = AS01 (976bp)
Lane 4 = AS02 (514bp
Lane 5 = AS03 (444bp)
Bands are at correct sizes; the PCR reaction was successful.
The three bands were cut out, placed in a 1.5mL microcentrifuge tube, and resuspended in 600uL of ADB buffer.
PCR product mixture was stored until 2.24.2010.
2.24.2010
Overall Progress
PCA Amplification reaction for sbb12 was successful and the product was purified using Small-Frag Zymo Cleanup.
Performed Zymo Gel Purification and second PCR reaction for sbb14
sbb12 progress: Design --> Assembly --> Amplification --> Zymo Clean-up --> Restriction Digest --> Preparative Gel --> Cloning
sbb14 progress: Initial PCR Reactions --> Preparative Gel --> Zymo Gel Purification --> Secondary PCR Reactions--> Analytical Gel --> Restriction Digest --> Cloning
sbb12
Analytical gel was run for PCA amplification product.

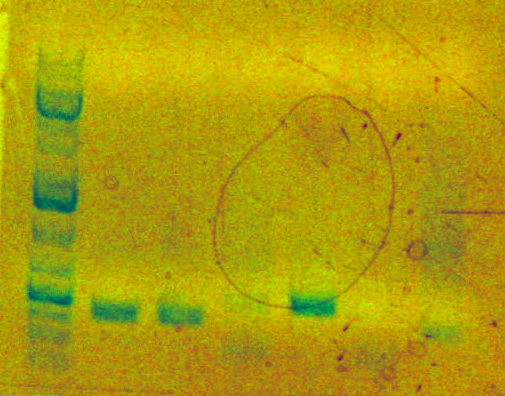
Lane 4 = AS04 (259bp)
Smearing is expected with PCA, but a band nevertheless appears at the correct size.
Amplification was deemed a success and a Small-Frag Zymo Cleanup was performed on the amplification product.
Restriction digest with EcoR1/BamH1 will be performed Friday, 2.26.2010.
sbb14
PCR Product mixture was purified using Zymo Gel Purification.
Purified fragments were eluted in 50uL of ddH2O.
Tube was labeled AS123.
Second round of PCR was then set up using the purified mixture of fragments as template DNA.
AS009-F and AS012-R were used as the oligos for the PCR.
The following PCR protocol was used:
24uL ddH2O 3.3uL 10x Expand Buffer "2" 3.3uL dNTPs (2mM in each) 1uL Oligo 1, 10uM (AS009-F) 1uL Oligo 2, 10uM (AS012-R) 0.5uL Expand polymerase "1" 0.5uL Template DNA (AS123)
2.26.2010
Overall Progress
Digested sbb12 with EcoR1/BamH1 and ran the product on a preparative gel.
Ran product of second round of PCR for sbb14 on an analytical gel.
sbb12 progress: Design --> Assembly --> Amplification --> Zymo Clean-up --> Restriction Digest --> Preparative Gel --> Cloning
sbb14 progress: Initial PCR Reactions --> Preparative Gel --> Zymo Gel Purification --> Secondary PCR Reactions --> Analytical Gel --> Restriction Digest --> Cloning
sbb12
Performed EcoR1/BamH1 restriction digest on cleaned up PCA amplification product according to the following protocol:
8uL of eluted PCR product 1uL of NEB Buffer 2 0.5uL EcoRI 0.5uL BamHI
Reaction mixture was then placed in a thermocycler at 37C for 1 hour.
After thermocycling, a mixture of 6uL of product and 4uL of loading buffer was run on a preparative gel.

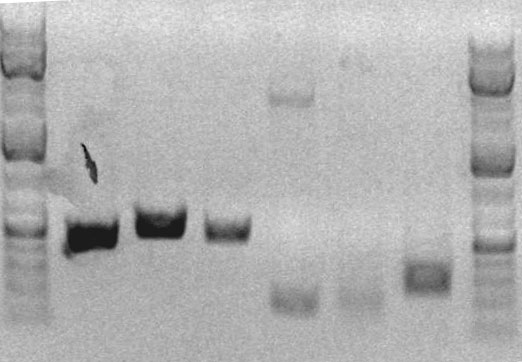
Lane 7 = AS04 (259bp)
The appropriate band was then cut out of the gel and resuspended in 600uL of ADB buffer for weekend storage.
sbb14
Product of the second round of PCR was run on an analytical gel.


Lane 6 = AS123 (1868bp)
No band was observed in Lane 6. SOEing failed.
Second round of PCR reactions will be repeated Monday, 3.1.2010.
3.1.2010
Overall Progress
Purified, ligated and transformed sbb12.
Ran second round of SOEing PCR reactions again using added DMSO and a different thermocycling protocol in an attempt to troubleshoot failed SOEing.
sbb12 progress: Design --> Assembly --> Amplification --> Zymo Clean-up --> Restriction Digest --> Preparative Gel --> Zymo Gel Purification --> Transformation --> Miniprep
sbb14 progress: Initial PCR Reactions --> Preparative Gel --> Zymo Gel Purification --> Secondary PCR Reactions --> Analytical Gel --> Secondary PCR Reactions (initial set failed) --> Restriction Digest --> Transformation --> Miniprep
sbb12
The band cut out of the preparative gel was purified by Zymo Gel Purification.
Digested/purified pBjk2741-Bca1144 was provided.
The purified fragment and vector backbone were then ligated according to the following protocol:
6.5uL ddH2O
1uL T4 DNA Ligase Buffer (small red or black-striped tubes)
1uL Vector digest (AS04)
1uL Insert digest (pBjk2741-Bca1144)
0.5uL T4 DNA Ligase
* Pound upside down on the bench to mix
* Give it a quick spin to send it back to the bottom of the tube
* Incubate on the benchtop for 30min
Product of ligation reaction was then transformed into competent cells.
The following transformation protocol was used:
1. Thaw a 200 uL aliquot of cells on ice 2. Add 50 uL of water to the cells 3. Add 30 uL of KCM to the cells 4. Put your ligation mixture on ice, let cool a minute or two 5. Add 70 uL of the cell cocktail to the ligation, stir to mix 6. Let sit on ice for 10 min 7. Heat shock for 90 seconds at 42 (longer incubation may work better) 8. Put back on ice for 1 min 9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour (LB Broth used) 10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight
JTK086 Competent Cells were used.
Transformed cells were then plated by GSIs.
sbb14
Second round of PCR reactions repeated according to the following protocol:
24uL ddH2O (substitute: 3.3uL DMSO/20.7uL ddH2O) 3.3uL 10x Expand Buffer "2" 3.3uL dNTPs (2mM in each) 1uL Oligo 1, 10uM (AS009-F) 1uL Oligo 2, 10uM (AS012-R) 0.5uL Expand polymerase "1" 0.5uL Template DNA (AS123)
3.3uL DMSO/20.7uL ddH2O was used instead of 24uL ddH2O.
Also, 2K45 was used as the thermocycling protocol rather than 2K55.
3.3.2010
Overall Progress
Performed a MiniPrep on the sbb12 colonies.
Ran analytical gel for second attempt at the second round of SOEing PCR reactions for sbb14.
sbb12 progress: Design --> Assembly --> Amplification --> Zymo Clean-up --> Restriction Digest --> Preparative Gel --> Zymo Gel Purification --> Transformation --> Miniprep --> Mapping
sbb14 progress: Initial PCR Reactions --> Preparative Gel --> Zymo Gel Purification --> Secondary PCR Reactions --> Analytical Gel --> Secondary PCR Reactions (initial set failed) --> Restriction Digest --> Transformation --> Miniprep --> Mapping
sbb12
Pelleted 4 colonies which had been grown in LB for 24h.
Plasmid DNA was then extracted from each colony by Macherey-Nagel Miniprep
The extracted plasmid DNA was then eluted into 4 tubes labeled:
1. AS sbb12 Clone 1 2. AS sbb12 Clone 2 3. AS sbb12 Clone 3 4. AS sbb12 Clone 4
Mapping will be performed Friday, 3.5.2010.
sbb14
Ran analytical gel for second attempt at the second round of SOEing PCR reactions.


Lane 12 = AS123 (1868bp)
The band is faint, but is nevertheless present at the correct size.
On Friday, 3.5.2010, the sample will be digested with EcoR1/BamH1 and run on a preparative gel.
3.8.2010
Overall Progress
Performed analytical mapping digest followed by an analytical gel for sbb12.
Performed a Zymo Cleanup followed by a restriction digest and preparative gel for sbb14.
sbb12 progress: Design --> Assembly --> Amplification --> Zymo Clean-up --> Restriction Digest --> Preparative Gel --> Zymo Gel Purification --> Transformation --> Miniprep --> Mapping --> Sequencing
sbb14 progress: Initial PCR Reactions --> Preparative Gel --> Zymo Gel Purification --> Secondary PCR Reactions --> Analytical Gel --> Secondary PCR Reactions (initial set failed) --> Zymo Cleanup --> Restriction Digest --> Preparative Gel --> Zymo Gel Purification --> Transformation --> Miniprep --> Mapping --> Sequencing
sbb12
Analytical mapping digest performed according to the following protocol:
Set up the following 10uL reaction in a PCR tube:
4uL ddH2O
4uL Miniprepped plasmid
1uL 10x NEB Buffer 2
0.5uL EcoRI
0.5uL BamHI (for parts >250bp) or XhoI (for parts <250bp)
* Incubate at 37 on the thermocycler for 30 minutes
An analytical gel was then run on the product:



Lane 1: Ladder
Lane 2: sbb12 Clone 1
Lane 3: sbb12 Clone 2
Lane 4: sbb12 Clone 3
Lane 5: sbb12 Clone 4
Lane 6: Ladder
Bands appeared at the the appropriate length for vector+insert (2170+259bp) and at the appropriate length for just the insert (259bp).
Second gel image has been doctored to bring out the faint bands at 259bp.
All 4 clones were then sent out for sequencing.
sbb14
A Zymo cleanup was performed on the secondary PCR reactions followed by a restriction digest with EcoR1/BamH1 using the following protocols:
1. Add 180 uL of Zymo ADB buffer (brown bottle) to a 33uL or 50uL reaction. 2. Transfer into the Zymo column (small clear guys) 3. spin through, discard waste. 4. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 5. spin through, discard waste. 6. Add 200 uL of PE or Zymo Wash buffer 7. spin through, discard waste. 8. spin for 90 seconds, full speed to dry. 9. elute with water into a fresh Eppendorf tube, use the same volume of water as the volume of the original reaction
* Set up the following reaction:
8uL of eluted PCR product
1uL of NEB Buffer 2
0.5uL EcoRI
0.5uL BamHI
* Incubate at 37 degrees on the thermocycler for 1hr
Product was then run on a preparative gel:


Lane 1: Ladder
Lane 2: sbb14 (1868bp)
The appropriate band was then cut out and stored in 600uL ADB Buffer.
3.10.2010
Overall Progress
Analyzed sequence data for clones 1 and 2 for sbb12.
Performed a Zymo Gel Purification followed by ligation and transformation for sbb14.
(*COMPLETE*) sbb12 progress: Design --> Assembly --> Amplification --> Zymo Clean-up --> Restriction Digest --> Preparative Gel --> Zymo Gel Purification --> Transformation --> Miniprep --> Mapping --> Sequencing
sbb14 progress: Initial PCR Reactions --> Preparative Gel --> Zymo Gel Purification --> Secondary PCR Reactions --> Analytical Gel --> Secondary PCR Reactions (initial set failed) --> Zymo Cleanup --> Restriction Digest --> Preparative Gel --> Zymo Gel Purification --> Transformation --> Miniprep --> Mapping --> Sequencing
sbb12 (*COMPLETE*)
The sequencing data for clone 1 was perfect except for a base insertion in the BglII site (agaTtct).
Clone 2 was perfect aside from a point mutation in the EcoR1 site (gGattc).
sbb14
Performed a Zymo Gel Purification on the digested product of second PCR reactions.
Product was then ligated into vector pBjk2741 using the following protocol to form pBjk2741-sbb14.
* Set up the following reaction:
6.5uL ddH2O
1uL T4 DNA Ligase Buffer (small red or black-striped tubes)
1uL Vector digest
1uL Insert digest
0.5uL T4 DNA Ligase
* Pound upside down on the bench to mix
* Give it a quick spin to send it back to the bottom of the tube
* Incubate on the benchtop for 30min
* Put on ice and proceed to the transformation
Ligation product was then transformed into competent JTK086 cells according the following protocol and plated overnight.
1. Thaw a 200 uL aliquot of cells on ice 2. Add 50 uL of water to the cells 3. Add 30 uL of KCM to the cells 4. Put your ligation mixture on ice, let cool a minute or two 5. Add 70 uL of the cell cocktail to the ligation, stir to mix 6. Let sit on ice for 10 min 7. Heat shock for 90 seconds at 42 (longer incubation may work better) 8. Put back on ice for 1 min 9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour 10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight