SBB10Ntbk-Jeni Lee: Difference between revisions
Jennifer Lee (talk | contribs) |
Jennifer Lee (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
[[Template:SBB10Project-19395]] <br> | [[Template:SBB10Project-19395]] <br> | ||
==3/17/2010== | |||
'''Ligation and transformation for tn5'''<br> | |||
Ligation for EcoRI/BamHI via [[Template:SBB-Protocols_Enz4]], transformation per [[Template:SBB-Protocols_Micro1]]. <br> | |||
==3/15/2010== | ==3/15/2010== | ||
'''Analytical Gel for tn5, zymo cleanup'''<br> | '''Analytical Gel for tn5, zymo cleanup'''<br> | ||
tn5 part:<br> | tn5 part:<br> | ||
*Zymo cleanup: [[Template:SBB-Protocols_Zymo1]].<br> | *Zymo cleanup: [[Template:SBB-Protocols_Zymo1]].<br> | ||
==3/10/2010== | ==3/10/2010== | ||
| Line 10: | Line 14: | ||
'''Miniprep for Ala6 & second round PCR for tn5'''<br> | '''Miniprep for Ala6 & second round PCR for tn5'''<br> | ||
Ala6 part:<br> | Ala6 part:<br> | ||
*Macherey-Nagel miniprep protocol via [[Template:SBB-Protocols_Micro4]]. <br> | *Macherey-Nagel miniprep protocol via [[Template:SBB-Protocols_Micro4]]. Used 2.5mL of each clone to generate a sizeable pellet.<br> | ||
*Verify plasmid sizes via [[Template:SBB-Protocols_Enz6]]. Used Xho1, as the part is <250bp.<br> | *Verify plasmid sizes via [[Template:SBB-Protocols_Enz6]]. Used Xho1, as the part is <250bp.<br> | ||
tn5 part:<br> | tn5 part:<br> | ||
Revision as of 16:16, 10 March 2010
3/17/2010
Ligation and transformation for tn5
Ligation for EcoRI/BamHI via Template:SBB-Protocols_Enz4, transformation per Template:SBB-Protocols_Micro1.
3/15/2010
Analytical Gel for tn5, zymo cleanup
tn5 part:
- Zymo cleanup: Template:SBB-Protocols_Zymo1.
3/10/2010
--Jennifer Lee 18:01, 10 March 2010 (EST)
Miniprep for Ala6 & second round PCR for tn5
Ala6 part:
- Macherey-Nagel miniprep protocol via Template:SBB-Protocols_Micro4. Used 2.5mL of each clone to generate a sizeable pellet.
- Verify plasmid sizes via Template:SBB-Protocols_Enz6. Used Xho1, as the part is <250bp.
tn5 part:
- PCR via Template:SBB-Protocols_PCR2.
3/8/2010
--Jennifer Lee 18:47, 8 March 2010 (EST)
Ligation & transformation for Ala6 & (failed) EcoRI/BamHI digestion for tn5
Set up digestion first and run ligation/transformation reaction within the hour-long digestion incubation.
Ala6 part:
- Ligation and transformation via Template:SBB-Protocols_Enz5 and Template:SBB-Protocols_Micro1.Plated on specR plate.
tn5 part:
- EcoRI/BamHI digestion via Template:SBB-Protocols_Enz2.

In lane 10 (sbb07) the fragment is approximately ~500 bp, which is not the size desired (should have been ~1450). Going back to the gel ran for 3/5/2010, it is evident the product was already wrong before the digest. Redoing the digest for sbb 07 and hopefully correcting this problem by re-diluting the oligos. It appears sbb07 1/3 were used instead of 1/2 for the second round of PCR.
3/3/2010
--Jennifer Lee 18:15, 3 March 2010 (EST)
*Ligation and transformation* for Ala6 part & analytical gel for tn5 part
Ala6 part:
- Ligation reaction protocol for EIPCR products via Template:SBB-Protocols_Enz5. Ala6 part is already in pBjk2741 vector so no additional vector is added. Transformation by heat-shock into EColi protocol via Template:SBB-Protocols_Micro1. Cell strain JTK086, marked with double blue stripe.
- Discovered the cell cocktail for ligation was done incorrectly; must re-start ligation/transformation reaction next week.*
tn5 part:
- Run analytical gel for tn5 (3uL PCR product + 7uL loading buffer).
- Gel Result:

- Lanes 6/7: sbb07 1/2 DMSO & sbb07 1/2
- Indicates successful SOEing reaction at new program, 2K45. Running zymo cleanup of sbb07 post PCR product (only the sbb07 1/2 reaction, stored the sbb07 1/2 DMSO reaction). Tube for sbb07 template is labelled "sbb07/temp post zymo for digest".
3/1/2010
--Jennifer Lee 17:28, 1 March 2010 (EST)
PCR attempt 2 for tn5 & zymo cleanup for Ala6; no time for ligation/transformation
Usual zymo cleanup on Ala6 prep gel product (with agarose, had added 600uL ADB buffer at the end of last lab for storage). Stored in BioE140L Box A as "sbb34 post zymo full template for ligation".
Re-ran 2 PCRs for sbb07 (tn5), once normally as a second attempt, another with 3.3uL DMSO substituted for 3.3uL of the total 24uL water. Running PCRs with 2K45 program, labelled PCR tubes as "sbb07 1/2" and "sbb07 1/2 DMSO". Protocol via Template:SBB-Protocols_PCR3.
2/24/2010
--Jennifer Lee 16:26, 24 February 2010 (EST)
Analytical gel for PCR2 & digest for Ala6
Run analytical gel for second PCR round for tn5 (sbb07) (3uL PCR product + 7uL loading buffer). Named tn5,2 on analytical gel. Also run zymo cleanup via Template:SBB-Protocols_Zymo1 for the same sample. Store purified digest in "sbb07 post-zymo & PCR2" tube, placed in BiOE140L Box A.
- Analytical gel result:
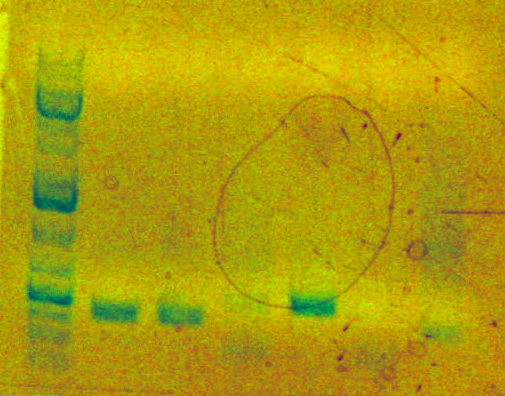 .
.
Expected a 1456bp length fragment which did not appear in Lane 7. Considering a different protocol for SOEing.
Tubes of DNA now labelled: (format: LID:SIDE)
sbb34: sbb34 post zymo cleanup (ready for digest)
sbb07 tn5: tn5 prep for PCR2 templates combined (before running PCR2, the two templates from PCR1 combined)
sbb07: post zymo& PCR2 (after running PCR2, ready for digest)
BglII EIPCR product digest of Ala6 (sbb34) via Template:SBB-Protocols_Enz3.
Incubated 2-3pm. Tube is labelled "sbb34 digest rxn, Ala6 digest". Ran gel with 10uL digestion product and 1uL loading buffer. Cut out band *SEE IMAGE*, labelled tube "sbb34 post digest: cut Ala6 post digest 2219bp" --> should be labelled 2203 bp
2/22/2010
--Jennifer Lee 16:14, 22 February 2010 (EST)
Last week: PCR
Cloned DNA sample using PCR protocol available Template:SBB-Protocols_PCR2
This week: Analytical & Preparative Gels & Zymo Cleanup; setup round 2 PCR
Preparative gel for SOEing reaction (tn5)
6uL PCR product + 4uL loading buffer
Tubes for gel labelled sbb071/3 and sbb072/4.
Analytical gel for Ala6
3uL PCR product + 7uL loading buffer
Tube for gel labelled sbb34
Ran Template:SBB-Protocols_Zymo1 for Ala6 part. Stored in BioE140L Box A.
Ran Template:SBB-Protocols_Zymo3 for Tn5. (Melted prep gel). Eluted DNA in 50uL water, stored DNA template in "tn5 PCR2 template combined" tube (lid says "sbb07, tn5"); stored in BioE140L Box A. Run new PCR for "Tn05 #2" according to Template:SBB-Protocols_PCR2. Stored round 1 PCR products in BioE140L Box A.
Procedure flow:
PCR --> Analytical Gel --> Zymo cleanup --> Digest --> Gel purification --> Ligation --> Transformation
2/10/2010
--Jennifer Lee 18:03, 10 February 2010 (EST)
Updated Construction files
Construction of Ala6 basic part sbb34
EIPCR Ala6-F/tn0002R on pBjk2741-Bca1144 (2219 bp, BglII)
Product is pBjk2741-sbb34 {<Ala6>}
----
Ala6-F EIPCR construction of Alanine-6 part
CCATAagatctgcTgcCgcagcagcagcaGGATCCtaaCTCGCTCCTCagg
tn0002R Reverse BglII oligo for Tn5 EIPCR
CCAATAGATCTcatgaattcCACTTCAG
Part: gatctgcTgcCgcagcagcagcaG
Construction of Tn5 transposase basic part sbb07 {<tn5>}
PCR tn5001/tn5003 on pRL27 (430 bp, gp = A)
PCR tn5004/tn5002 on pRL27 (1056 bp, gp = B)
---------------------------------------------------
PCR tn5001/tn5002 on A+B (1456 bp, EcoRI/BamHI)
Digest pBjk2741-Bca1144 (EcoRI/BamHI, 2170+910, L)
Product is pBjk2741-tn5
---------------------------------------------------
tn5001 Forward EcoRI and BglII for tn5
CCATAgaattcATGagatctATAACTTCTGCTCTTCATCGTG
tn5004 (F)Removing the Xho1 site in tn5
CGTTCTCTTGCTaGAGGCCACCACATTCCG
tn5003 (R)Removing the Xho1 site in tn5
CGGAATGTGGTGGCCTCtAGCAAGAGAACG
tn5002 Reverse BamHI and BglII removal for tn5
CTGATggatccGATCTTGATCCCCTGCGCC
Part: gatctATAACTTCTGCTCTTCATCGTGCGGCCGACTGGGCTAAATCTGTGTTCTCTTCGGCGGCGCTGGGTGATCCTCGCCGTACTGCCCGCTTGGTTAACGTCGCCGCCCAATTGGCAAAATATTCTGGTAAATCAATAACCATCTCATCAGAGGGTAGTAAAGCCGCCCAGGAAGGCGCTTACCGATTTATCCGCAATCCCAACGTTTCTGCCGAGGCGATCAGAAAGGCTGGCGCCATGCAAACAGTCAAGTTGGCTCAGGAGTTTCCCGAACTGCTGGCCATTGAGGACACCACCTCTTTGAGTTATCGCCACCAGGTCGCCGAAGAGCTTGGCAAGCTGGGCTCTATTCAGGATAAATCCCGCGGATGGTGGGTTCACTCCGTTCTCTTGCTaGAGGCCACCACATTCCGCACCGTAGGATTACTGCATCAGGAGTGGTGGATGCGCCCGGATGACCCTGCCGATGCGGATGAAAAGGAGAGTGGCAAATGGCTGGCAGCGGCCGCAACTAGCCGGTTACGCATGGGCAGCATGATGAGCAACGTGATTGCGGTCTGTGACCGCGAAGCCGATATTCATGCTTATCTGCAGGACAAACTGGCGCATAACGAGCGCTTCGTGGTGCGCTCCAAGCACCCACGCAAGGACGTAGAGTCTGGGTTGTATCTGTACGACCATCTGAAGAACCAACCGGAGTTGGGTGGCTATCAGATCAGCATTCCGCAAAAGGGCGTGGTGGATAAACGCGGTAAACGTAAAAATCGACCAGCCCGCAAGGCGAGCTTGAGCCTGCGCAGTGGGCGCATCACGCTAAAACAGGGGAATATCACGCTCAACGCGGTGCTGGCCGAGGAGATTAACCCGCCCAAGGGTGAGACCCCGTTGAAATGGTTGTTGCTGACCAGCGAACCGGTCGAGTCGCTAGCCCAAGCCTTGCGCGTCATCGACATTTATACCCATCGCTGGCGGATCGAGGAGTTCCATAAGGCATGGAAAACCGGAGCAGGAGCCGAGAGGCAACGCATGGAGGAGCCGGATAATCTGGAGCGGATGGTCTCGATCCTCTCGTTTGTTGCGGTCAGGCTGTTACAGCTCAGAGAAAGCTTCACGCCGCCGCAAGCACTCAGGGCGCAAGGGCTGCTAAAGGAAGCGGAACACGTAGAAAGCCAGTCCGCAGAAACGGTGCTGACCCCGGATGAATGTCAGCTACTGGGCTATCTGGACAAGGGAAAACGCAAGCGCAAAGAGAAAGCAGGTAGCTTGCAGTGGGCTTACATGGCGATAGCTAGACTGGGCGGTTTTATGGACAGCAAGCGAACCGGAATTGCCAGCTGGGGCGCCCTCTGGGAAGGTTGGGAAGCCCTGCAAAGTAAACTGGATGGCTTTCTTGCCGCCAAGGATCTGATGGCGCAGGGGATCAAGATCG
2/9/2010
--Jennifer Lee 00:45, 9 February 2010 (EST)
Completed project parts:
Construction of Tn5 transposase basic part sbb07 {<tn5>}
PCR tn5001/tn5003 on pRL27 (438 bp, gp = A)
PCR tn5004/tn5002 on pRL27 (1069 bp, gp = B)
---------------------------------------------------
PCR tn5001/tn5002 on A+B (1462 bp, EcoRI/BamHI)
Digest pBjk2741-Bca1144 (EcoRI/BamHI, 2170+910, L)
Product is pBjk2741-tn5
---------------------------------------------------
tn5001 Forward EcoRI and BglII for tn5 CCATAgaattcATGagatctATGATAACTTCTGCTCTTCATCGTGC
tn5004 (F)Removing the Xho1 site in tn5 GGGTTCACTCCGTTCTCTTGCTCGAAG
tn5003 (R)Removing the Xho1 site in tn5 CGGTGCGGAATGTGGTGGCTTCGAG
tn5002 Reverse BamHI and BglII removal for tn5 CTGATggatccTCGGATCTTGATCCCCTGCGCC
Construction of Ala6 basic part sbb34
EIPCR Ala6-F/ca2741R on pBjk2741-Bca1144 (2203 bp, BglII)
Product is pBjk2741-Ala6 {<Ala6>}
----
Ala6-F EIPCR construction of Alanine-6 part
CCATAagatctgcagcagcagcagcagcaGGATCCtaaCTCGCTCCTCagg
jl2741R Reverse BglII oligo for Ala6 EIPCR on pBjk2741-Bca1144
CTGAAGTGgaattcatgAGATCTATTGG