SBB10Ntbk-JennaKelleher: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
==3/3/2010== | ==3/3/2010== | ||
===Gameplan=== | ===Gameplan=== | ||
# If ligations can sit over the weekend (ask GSI), start [[Template:SBB-Protocols_Enz4 | Ligation of EcoRI/BamHI digests]] for sbb28, sbb29. Otherwise, proceed to (2). | # If ligations can sit over the weekend (ask GSI), start [[Template:SBB-Protocols_Enz4 | Ligation of EcoRI/BamHI digests]] for sbb28, sbb29 and then do [[Template:SBB-Protocols_Micro1 | Transformation by heat-shock]]. Otherwise, proceed to (2). | ||
# Perform [[Template:SBB-Protocols_Micro4 | Macherey-Nagel Miniprep]] for sbb42, either while waiting for ligations or on its own. | # Perform [[Template:SBB-Protocols_Micro4 | Macherey-Nagel Miniprep]] for sbb42, either while waiting for ligations or on its own. | ||
# Ask about homework. | # Ask about homework. | ||
===Lab Issues=== | ===Lab Issues=== | ||
sbb42 miniprep | sbb42 miniprep | ||
centrifuged instead of vortexed, pipetted 42-3 up and down instead of vortexing (vortexed the rest after...) | centrifuged instead of vortexed, pipetted 42-3 up and down instead of vortexing (vortexed the rest after...) | ||
42-4 DNA column seems sketchy... liquid making it to bottom of the tube before centrifuge | 42-4 DNA column seems sketchy... liquid making it to bottom of the tube before centrifuge | ||
DNA sat in columns in between miniprep steps for a while... up to 5 minutes. Hopefully this isn't a problem. | |||
sbb28 and sbb29 ligations | sbb28 and sbb29 ligations | ||
added LB (2YT) instead of KCM to cells before heat shocking - tossing samples and redoing ligation next week | added LB (2YT) instead of KCM to cells before heat shocking - tossing samples and redoing ligation next week | ||
| Line 17: | Line 17: | ||
- Ligation and Transformation of sbb28 and sbb29 <br> | - Ligation and Transformation of sbb28 and sbb29 <br> | ||
====Wednesday, 3/11/2010==== | ====Wednesday, 3/11/2010==== | ||
- Miniprep and Analytical Gel for sbb28 and sbb29 | - Miniprep and Analytical Gel for sbb28 and sbb29, then they'll be ready for sequencing | ||
==3/1/2010== | ==3/1/2010== | ||
Performed EcoRI/BamHI transfer for sbb42, followed by ligation, transformation, and rescue. <br> | Performed EcoRI/BamHI transfer for sbb42, followed by ligation, transformation, and rescue. <br> | ||
Revision as of 16:12, 3 March 2010
3/3/2010
Gameplan
- If ligations can sit over the weekend (ask GSI), start Ligation of EcoRI/BamHI digests for sbb28, sbb29 and then do Transformation by heat-shock. Otherwise, proceed to (2).
- Perform Macherey-Nagel Miniprep for sbb42, either while waiting for ligations or on its own.
- Ask about homework.
Lab Issues
sbb42 miniprep
centrifuged instead of vortexed, pipetted 42-3 up and down instead of vortexing (vortexed the rest after...) 42-4 DNA column seems sketchy... liquid making it to bottom of the tube before centrifuge DNA sat in columns in between miniprep steps for a while... up to 5 minutes. Hopefully this isn't a problem.
sbb28 and sbb29 ligations
added LB (2YT) instead of KCM to cells before heat shocking - tossing samples and redoing ligation next week
What's Next
Monday, 3/8/2010
- Analytical Gel for sbb42: If it worked, the samples will be ready for sequencing
- Ligation and Transformation of sbb28 and sbb29
Wednesday, 3/11/2010
- Miniprep and Analytical Gel for sbb28 and sbb29, then they'll be ready for sequencing
3/1/2010
Performed EcoRI/BamHI transfer for sbb42, followed by ligation, transformation, and rescue.
- Incubation was started at 3:55pm - plating done by GSI's (thank you!)
Meanwhile, zymo'd sbb28 and sbb29 preperative gel products - ready for ligation/transformation next time.
2/24/2010
Labwork
Performed EcoRI/BamHI Digests on sbb28 and sbb29
After, ran 2 preparative gels
10 microliters digestion product 1 microliters undiluted 6x loading buffer
Gel Results
Success! Gel loaded and imaged by Andrew Saarni

Relevant Lanes:
- Ladder
- blank (ran off the gel...)
- sbb28
- sbb29
After, cut out bands and melted in 600 microliters of ADB buffer, stored for later use.
2/22/2010
Labwork
Last time: basic PCR for sbb28, sbb29
This time: ran 2 analytical gels
3 microliters PCR 7 microliters Loading Buffer
While waiting, ran zymo cleanup on the 2 samples, final volume of 30.6 microliters
Stored in 140L Box A
Next time: digestion
Gel Results
Analytical gel images shown below (ladder breakdown on left, gels on right), loaded and imaged by Dorothy Tulanont

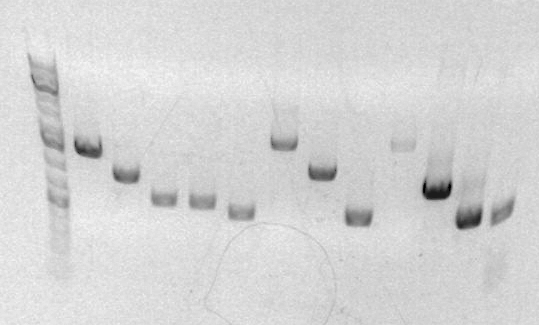
Relevant Lanes:
- ladder
- sbb28 - looks to be about 1500 bp
- sbb29 - looks to be about 1000 bp
Both of these values are close to desired lengths, so PCR worked!
2/17/2010
Labwork
Prepared sbb28 and sbb29 for PCR after creating a master mix with Jeni Lee
sbb28 in 2k55 and sbb29 in 55
Updated Constuction Files
Biobricking of 099 rep protein basic part sbb29
PCR repo99-F and JG002-R on pEC52 gene (978 bp, EcoRI/BamHI)
Sub into pBca9523-Bca1144 (EcoRI/BamHI, 910+2472, L)
Product is pBca9523-sbb29 {rbs.repO99!}
----------------------------
repo99-F Cloning of repo99
ccaaaGAATTCatgAGATCTcctggaaggaatcgtaacac
JG002-R Cloning of O99 rep Gene
CTGATGGATCCCTACTCTACAAGACCTCGTTTTttc
Biobricking of CA42 rep gene basic part sbb28
PCR repCA42-F and KRM002 on pEC22-CA42 gene (1496 bp, EcoRI/BamHI)
Sub into pBca9523-Bca1144 (EcoRI/BamHI, 910+2472, L)
Product is pBca9523-sbb28 {5'UTR.repCA42!}
----------------------------
repCA42-F Cloning of repCA42
ccaaaGAATTCatgAGATCTcagctgaagtgaccggattag
KRM002 Reverse oligo for cloning of rbs.repCA42!
ctgatGGATCCctattttccgcttttccag
Biobricking of pre-pro sequence basic part sbb42
Eco/Bam transfer pBjh1601AC-Bjh1723
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-Bjh1723 {rbs_pelB>}
2/10/2010
Project Information
29071-O99 rep Protein, CA42 rep Gene, Pre-Pro sequence
Construction Files
Jenna Kelleher 099 Rep Protein (sbb29)
Jenna Kelleher CA42 Rep Gene (sbb28)
Jenna Kelleher Pre-pro Sequence (sbb42)
Useful Protocols
For SBB28 and SBB29:
Cloning by PCR
Regular Zymo Cleanup
For SBB42:
Eco-Bam Transfer
Small-Frag Zymo Cleanup
For All 3:
EcoRI/BamHI Digest of PCR Products
Zymo Gel Purification
Ligation of EcoRI/BamHI digests
Transformation by heat-shock
Picking of colonies
Macherey-Nagel Miniprep
EcoRI/BamHI Digest of PCR Products
Analytical digests (Mapping)