SBB10Ntbk-JennaKelleher: Difference between revisions
No edit summary |
No edit summary |
||
| (23 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[http://openwetware.org/wiki/SynBioBootcamp:Notebook/SynBioBootcamp10#Notebooks Main page] <br> | [http://openwetware.org/wiki/SynBioBootcamp:Notebook/SynBioBootcamp10#Notebooks Main page] <br> | ||
==3/12/2010 and beyond== | |||
see [[Template:SBB10AssayTeam1 | Team 1: Toxicity and Expression]] for any information regarding the assays run by my team and I for the second half of the semester... | |||
==3/10/2010== | |||
===Labwork=== | |||
Contamination in plates for sbb28 and sbb29, colonies were chosen by GSI's but may be contaminated due to bad LB solution. <br> | |||
Miniprepped sbb28 and sbb29, and performed analytical gels on 4 clones of each. <br> | |||
===Analytical Gel=== | |||
Ran another analytical gel for mapping of sbb28 and sbb29 clones:<br> | |||
[[Image:Generuler1kbplus.jpg]] | |||
[[Image:JKgel2.jpg]]<br> | |||
Lanes: <br> | |||
#Ladder | |||
#sbb28-1 | |||
#sbb28-2 | |||
#sbb28-3 | |||
#sbb28-4 | |||
#sbb29-1 | |||
#sbb29-2 | |||
#sbb29-3 | |||
#sbb29-4 | |||
#Ladder | |||
==3/8/2010== | |||
Performed [[Template:SBB-Protocols_Enz6 | Analytical digests (Mapping)]] of sbb42 samples which were miniprepped last time. Used EcoRI and BamHI restriction enzymes (SHOULD have used EcoRI and XhoI because part is less than 250 base pairs...) <br> | |||
Ran an analytical gel myself with results shown below: <br> | |||
[[Image:Generuler1kbplus.jpg]] | |||
[[Image:JKgel.jpg]]<br> | |||
Lanes: | |||
#Ladder | |||
#JH8D | |||
#Jh6ABC | |||
#42-1 | |||
#42-2 | |||
#42-3 | |||
#42-4 | |||
#sbb6 digest | |||
#Ladder | |||
(42-1 through 42-4 are mine)<br> | |||
From the gel, you can see that 42-2 and 42-3 have two bands, one around 2000 and the other around 900. This indicates that these two colonies still contained the original vector insert, and not the sbb42 part (which is so short that it wouldn't be visible on the band). So, samples 1 and 4 were turned in for sequencing. <br> | |||
<br> | |||
Meanwhile, I performed ligations and transformations for sbb28 and sbb29. Instead of using the pBca9523 vector (which we were all out of), these 2 parts were put into a different vector, pBca1256. | |||
==3/3/2010== | |||
===Gameplan=== | |||
# If ligations can sit over the weekend (ask GSI), start [[Template:SBB-Protocols_Enz4 | Ligation of EcoRI/BamHI digests]] for sbb28, sbb29 and then do [[Template:SBB-Protocols_Micro1 | Transformation by heat-shock]]. Otherwise, proceed to (2). | |||
# Perform [[Template:SBB-Protocols_Micro4 | Macherey-Nagel Miniprep]] for sbb42, either while waiting for ligations or on its own. | |||
# Ask about homework. | |||
===Lab Issues=== | |||
sbb42 miniprep | |||
centrifuged instead of vortexed, pipetted 42-3 up and down instead of vortexing (vortexed the rest after...) | |||
42-4 DNA column seems sketchy... liquid making it to bottom of the tube before centrifuge | |||
DNA sat in columns in between miniprep steps for a while... up to 5 minutes. Hopefully this isn't a problem. | |||
sbb28 and sbb29 ligations | |||
added LB (2YT) instead of KCM to cells before heat shocking - tossing samples and redoing ligation next week | |||
===What's Next=== | |||
====Monday, 3/8/2010==== | |||
- Analytical Gel for sbb42: If it worked, the samples will be ready for sequencing <br> | |||
- Ligation and Transformation of sbb28 and sbb29 <br> | |||
====Wednesday, 3/11/2010==== | |||
- Miniprep and Analytical Gel for sbb28 and sbb29, then they'll be ready for sequencing | |||
==3/1/2010== | |||
Performed EcoRI/BamHI transfer for sbb42, followed by ligation, transformation, and rescue. <br> | |||
- Incubation was started at 3:55pm - plating done by GSI's (thank you!) <br> | |||
<br> | |||
Meanwhile, zymo'd sbb28 and sbb29 preperative gel products - ready for ligation/transformation next time. | |||
<br> | |||
==2/24/2010== | ==2/24/2010== | ||
===Labwork=== | ===Labwork=== | ||
Latest revision as of 22:08, 3 May 2010
3/12/2010 and beyond
see Team 1: Toxicity and Expression for any information regarding the assays run by my team and I for the second half of the semester...
3/10/2010
Labwork
Contamination in plates for sbb28 and sbb29, colonies were chosen by GSI's but may be contaminated due to bad LB solution.
Miniprepped sbb28 and sbb29, and performed analytical gels on 4 clones of each.
Analytical Gel
Ran another analytical gel for mapping of sbb28 and sbb29 clones:

Lanes:
- Ladder
- sbb28-1
- sbb28-2
- sbb28-3
- sbb28-4
- sbb29-1
- sbb29-2
- sbb29-3
- sbb29-4
- Ladder
3/8/2010
Performed Analytical digests (Mapping) of sbb42 samples which were miniprepped last time. Used EcoRI and BamHI restriction enzymes (SHOULD have used EcoRI and XhoI because part is less than 250 base pairs...)
Ran an analytical gel myself with results shown below:


Lanes:
- Ladder
- JH8D
- Jh6ABC
- 42-1
- 42-2
- 42-3
- 42-4
- sbb6 digest
- Ladder
(42-1 through 42-4 are mine)
From the gel, you can see that 42-2 and 42-3 have two bands, one around 2000 and the other around 900. This indicates that these two colonies still contained the original vector insert, and not the sbb42 part (which is so short that it wouldn't be visible on the band). So, samples 1 and 4 were turned in for sequencing.
Meanwhile, I performed ligations and transformations for sbb28 and sbb29. Instead of using the pBca9523 vector (which we were all out of), these 2 parts were put into a different vector, pBca1256.
3/3/2010
Gameplan
- If ligations can sit over the weekend (ask GSI), start Ligation of EcoRI/BamHI digests for sbb28, sbb29 and then do Transformation by heat-shock. Otherwise, proceed to (2).
- Perform Macherey-Nagel Miniprep for sbb42, either while waiting for ligations or on its own.
- Ask about homework.
Lab Issues
sbb42 miniprep
centrifuged instead of vortexed, pipetted 42-3 up and down instead of vortexing (vortexed the rest after...) 42-4 DNA column seems sketchy... liquid making it to bottom of the tube before centrifuge DNA sat in columns in between miniprep steps for a while... up to 5 minutes. Hopefully this isn't a problem.
sbb28 and sbb29 ligations
added LB (2YT) instead of KCM to cells before heat shocking - tossing samples and redoing ligation next week
What's Next
Monday, 3/8/2010
- Analytical Gel for sbb42: If it worked, the samples will be ready for sequencing
- Ligation and Transformation of sbb28 and sbb29
Wednesday, 3/11/2010
- Miniprep and Analytical Gel for sbb28 and sbb29, then they'll be ready for sequencing
3/1/2010
Performed EcoRI/BamHI transfer for sbb42, followed by ligation, transformation, and rescue.
- Incubation was started at 3:55pm - plating done by GSI's (thank you!)
Meanwhile, zymo'd sbb28 and sbb29 preperative gel products - ready for ligation/transformation next time.
2/24/2010
Labwork
Performed EcoRI/BamHI Digests on sbb28 and sbb29
After, ran 2 preparative gels
10 microliters digestion product 1 microliters undiluted 6x loading buffer
Gel Results
Success! Gel loaded and imaged by Andrew Saarni


Relevant Lanes:
- Ladder
- blank (ran off the gel...)
- sbb28
- sbb29
After, cut out bands and melted in 600 microliters of ADB buffer, stored for later use.
2/22/2010
Labwork
Last time: basic PCR for sbb28, sbb29
This time: ran 2 analytical gels
3 microliters PCR 7 microliters Loading Buffer
While waiting, ran zymo cleanup on the 2 samples, final volume of 30.6 microliters
Stored in 140L Box A
Next time: digestion
Gel Results
Analytical gel images shown below (ladder breakdown on left, gels on right), loaded and imaged by Dorothy Tulanont

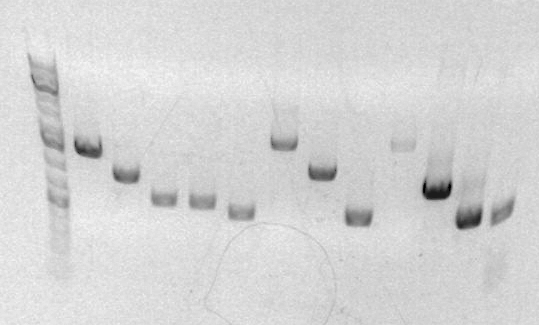
Relevant Lanes:
- ladder
- sbb28 - looks to be about 1500 bp
- sbb29 - looks to be about 1000 bp
Both of these values are close to desired lengths, so PCR worked!
2/17/2010
Labwork
Prepared sbb28 and sbb29 for PCR after creating a master mix with Jeni Lee
sbb28 in 2k55 and sbb29 in 55
Updated Constuction Files
Biobricking of 099 rep protein basic part sbb29
PCR repo99-F and JG002-R on pEC52 gene (978 bp, EcoRI/BamHI)
Sub into pBca9523-Bca1144 (EcoRI/BamHI, 910+2472, L)
Product is pBca9523-sbb29 {rbs.repO99!}
----------------------------
repo99-F Cloning of repo99
ccaaaGAATTCatgAGATCTcctggaaggaatcgtaacac
JG002-R Cloning of O99 rep Gene
CTGATGGATCCCTACTCTACAAGACCTCGTTTTttc
Biobricking of CA42 rep gene basic part sbb28
PCR repCA42-F and KRM002 on pEC22-CA42 gene (1496 bp, EcoRI/BamHI)
Sub into pBca9523-Bca1144 (EcoRI/BamHI, 910+2472, L)
Product is pBca9523-sbb28 {5'UTR.repCA42!}
----------------------------
repCA42-F Cloning of repCA42
ccaaaGAATTCatgAGATCTcagctgaagtgaccggattag
KRM002 Reverse oligo for cloning of rbs.repCA42!
ctgatGGATCCctattttccgcttttccag
Biobricking of pre-pro sequence basic part sbb42
Eco/Bam transfer pBjh1601AC-Bjh1723
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-Bjh1723 {rbs_pelB>}
2/10/2010
Project Information
29071-O99 rep Protein, CA42 rep Gene, Pre-Pro sequence
Construction Files
Jenna Kelleher 099 Rep Protein (sbb29)
Jenna Kelleher CA42 Rep Gene (sbb28)
Jenna Kelleher Pre-pro Sequence (sbb42)
Useful Protocols
For SBB28 and SBB29:
Cloning by PCR
Regular Zymo Cleanup
For SBB42:
Eco-Bam Transfer
Small-Frag Zymo Cleanup
For All 3:
EcoRI/BamHI Digest of PCR Products
Zymo Gel Purification
Ligation of EcoRI/BamHI digests
Transformation by heat-shock
Picking of colonies
Macherey-Nagel Miniprep
EcoRI/BamHI Digest of PCR Products
Analytical digests (Mapping)