SBB10Ntbk-JoannaChen
Joanna Chen 16:37, 17 March 2010 (EDT)
Sequencing data for the first two clones of both sbb17 and sbb18 came in last week. I analyzed the data and found 2 non-silent point mutations in sbb17 clone 1 and a deletion in sbb17 clone 2, so I needed additional clones of sbb17 sequenced. Both clone 1 and clone 2 of sbb18 were perfect.
Today, additional sequencing data for sbb17 came in. Both clones 3 and 4 were perfect.
Joanna Chen 17:17, 10 March 2010 (EST)
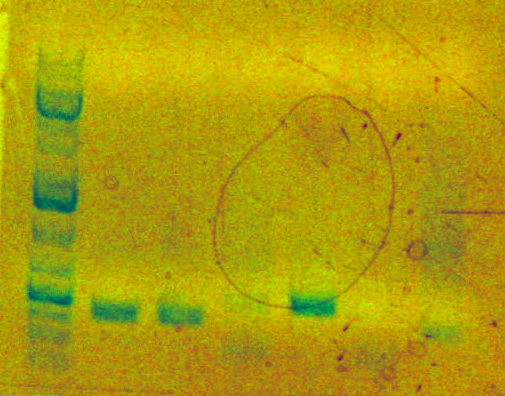
Today I performed analytical digests for mapping of the 4 clones of each of my 2 parts (sbb17 and sbb18).
Here is the gel picture. The first lane is the ladder, the next four are sbb17 clones 1-4, and the following four are sbb18 clones 1-4.

Fragments should be of size 408 bp and 2170 bp for both parts. They look correct. (The 408 bp bands are somewhat hard to see.)
Samples were then recorded in Clotho and transferred to the class plates.
Part Clone Plate Well sbb17 1 A A3 sbb17 2 A B3 sbb17 3 B A3 sbb17 4 B B3 sbb18 1 A C3 sbb18 2 A D3 sbb18 3 B C3 sbb18 4 B D3
Joanna Chen 01:08, 9 March 2010 (EST)
The GSI's picked 4 colonies from each of my 2 plates and grew the cells in liquid media before class. Today I did minipreps to isolate plasmid DNA from the cells. I pelleted cells from 3 mL of culture. Also, some of the buffers used were different but equivalent buffers, as indicated below:
- P2 = A2
- N3/P3 = A3
- PB = AW
- PE = A4
Tubes are labeled pBjk2741-sbb17 Clones 1 through 4 and pBjk2741-sbb18 Clones 1 through 4 (with today's date - 3/8/10 - on the side) and are placed in the box containing minipreped DNA.
Joanna Chen 18:28, 3 March 2010 (EST)
Today I finished the gel purification on the gels I cut on Monday. The purified EcoRI/BamHI digest products are in tubes labeled "sbb17 E/Ba gp JC 3/3" and "sbb18 E/Ba gp JC 3/3" in Box C. I then proceeded to ligation and transformation. For the rescue step, the cells were in the incubator for slightly less than an hour (about 50-55 mins) because I needed to leave for class. Plates were labeled pBjk2741-sbb17 and pBjk2741-sbb18.
Joanna Chen 18:12, 1 March 2010 (EST)
Today I started with an EcoRI/BamHI Digest on each of the PCR products from my gene synthesis. After incubating, I added 1 ul of 6x loading dye to each tube and waited for someone to run the gel. Here is the gel picture; sbb17 is on the far right, sbb18 is the second from the right.
I then tried my best to cut out the bands located near 424 bases. It was hard to see where the bands were located when I was cutting. Then I placed each piece of cut gel in the corresponding tube and added 600 ul ADB buffer to each sample and melted the gel. The tubes are labeled "sbb17 E/Ba Digest Gel JC 3/1" and "sbb18 E/Ba Digest Gel JC 3/1" and placed in Box C.
Joanna Chen 18:56, 24 February 2010 (EST)
First I prepared a small sample of the PCA amplification product for an analytical gel by combining 3ul of the product with 7ul of loading dye for each of sbb17 and sbb18. I then ran a gel for these samples and a few samples from other people. The first lane of the gel picture below is the ladder, the next two are sbb17 and sbb18 respectively. I was expecting a PCA product of 424 bases for both parts. The gel looks good.
Next, I did a Zymo cleanup on the PCA amplification product for each of my parts, using A4 wash buffer in place of PE and eluting with 50ul water. The purified products are in tubes labeled "sbb17 PCR Zymo 2/24 JC" and "sbb18 PCR Zymo 2/24 JC" in Box C.
Joanna Chen 18:06, 22 February 2010 (EST)
I did a Zymo cleanup on the product of the assembly reaction for each of my parts, using A4 wash buffer in place of PE and eluting with 50ul water. The purified products are in tubes labeled "sbb17 PCA Asbly Zymo 2/22/10 JC" and "sbb18 PCA Asbly Zymo 2/22/10 JC" in Box C.
Then, I set up the amplification reaction by combining:
1. 32.5 ul ddH2O 2. 10 ul 5x phusion buffer 3. 5 ul 2mM dNTPs 4. 1 ul purified assembly reaction product 5. 1 ul each outer oligo 6. .5 ul phusion
These are in PCA tubes labeled "sbb17 PCA Ampl." and "sbb18 PCA Ampl." and placed in the ice bucket for PCA.
Joanna Chen 17:25, 17 February 2010 (EST)
Today was the first day of wet lab.
I obtained the tubes containing the oligo mixes and set up the assembly portion of PCA for both of my parts (sbb17 and sbb18). I combined:
1. 38 uL ddH2O 2. 5 ul 10x expand buffer 3. 5 ul 2mM dNTPs 4. 1 ul oligo mixture 5. 0.75 ul Expand polymerase
PCA tubes are labeled "sbb17 PCA" and "sbb18 PCA" respectively, and placed into the ice bucket for PCA.
Last week
I designed my project parts for two similar zinc finger domains, (zf+) and (zf-), called sbb17 and sbb18 respectively. I aligned the two sequences using protein BLAST to find out where the sequences differed. Originally, I used GeneDesign on both sequences to generate oligos of target length 50bp, and then adjusted the ends of some oligos to increase the number of oligos that were the same in sbb17 and sbb18. However, this did not appear to work due to internal homology in the zinc finger domains. Later, Prof. Anderson provided me with a piece of code that generated nucleotide sequences that minimized internal repeats. I modified this code to first generate a nucleotide sequence for one of the zinc finger domains, and then generate the nucleotide sequence for the other zinc finger domain based on the sequence of the first and changing only what was necessary to produce the correct amino acid sequence. I then used GeneDesign to create oligos for these nucleotide sequences, and adjusted the ends of some oligos to increase the number of oligos that were the same in sbb17 and sbb18. In silico tests indicated that these oligos would assemble.