SBB10Ntbk-JoseGutierrez: Difference between revisions
No edit summary |
|||
| (21 intermediate revisions by the same user not shown) | |||
| Line 37: | Line 37: | ||
Procedure: | Procedure: | ||
#convert Oligos to 100uM [X(nmol) + 10*X(ul)] | #convert Oligos to 100uM [X(nmol) + 10*X(ul)] | ||
# dilute stock Oligos to 10uM | #dilute stock Oligos to 10uM | ||
#PCR Procedure [[Template:SBB- | #[[Template:SBB-Protocols_PCR2 | PCR Procedure]] | ||
==2/17/10== | |||
Objective: Run Analytical gel on SBB30 (JG01), after confirmation run zymo cleanup <br> | |||
Expected Length (1235 bp)<br> | |||
Results: | |||
===Dorothy=== | |||
[[Image:DDT_02_22_10.jpg]] [[Image:Generuler1kbplus.jpg]]<br> | |||
Lanes | |||
#ladder | |||
#28 | |||
#29 | |||
#sbb25 | |||
#sbb26 | |||
#sbb32 | |||
#sbb34 | |||
#JG1 | |||
#ZHH 1,2 AG | |||
#TN 001/002 PT | |||
#JHA | |||
#JHB | |||
#JHC | |||
PCR product confirmed, continue to [[Template:SBB-Protocols_Zymo1 | Zymo Cleanup]] | |||
==2/24/10== | |||
[[Template:SBB-Protocols_Enz2 | Eco/Bam Digest]] of SBB30 PCR products | |||
==3/1/10== | |||
To Do List: <br> | |||
[[Template:SBB-Protocols_Zymo3 | Zymo gell]] purification of SBB30 Eco/Bam Digest | |||
[[Template:SBB-EcoBamXfers | Eco/Bam transfer]] pBjh1601CK-ig114<br> | |||
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)<br> | |||
Product is pBjk2741-ig114 {AraC-Pbad} | |||
[[Template:SBB-EcoBamXfers | Eco/Bam transfer]] pBca1100-Bca1152<br> | |||
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)<br> | |||
Product is pBjk2741-Bca1152 {Pcon-J23100} | |||
Ligation's:<br> | |||
SBB30 (vector:pBca9523, start: 2:35, end: 3:07)<br> | |||
SBB38 (vector:pBjk2741, start: 3:10, end: 3:40)<br> | |||
SBB37 (vector:pBjk2741, start: 3:10, end: 3:40)<br> | |||
[[Template:SBB-Protocols_Micro1 | Transformation]] of Ligation products into competent cells | |||
==3/3/10== | |||
pick colonies (already done) | |||
*Note: SBB 37 was mostly red colonies on plate only two possible good colonies on plate, probably due to re-ligation of original vector also SBB37 is small which might make Eco/Bam transfer fail, other plates looked good | |||
[[Template:SBB-Protocols_Micro3 | Mini Prep]] picked colonies (4 SBB30, 4 SBB38, 2 SBB37) from plates | |||
==3/8/10== | |||
To Do: Run analytical gel of miniprep plasmids<br> | |||
-[[Template:SBB-Protocols_Enz2 | Eco/Bam Digest]] of miniprep plasmids<br> | |||
-run analytical get of digests, Expected product lengths: SBB30(1235 bp); SBB 38 (1253, 2170 bp); SBB37(2170, 50 bp)<br> | |||
Results: | |||
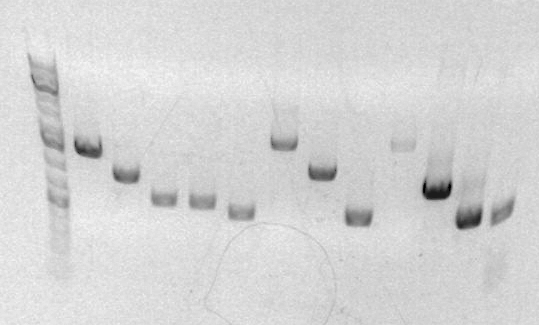
===Jose Gutierrez=== | |||
#JG1a(SBB30) | |||
#JG1b | |||
#JG1c | |||
#JG1d | |||
#JG2a(SBB 38) | |||
#JG2b | |||
#Ladder | |||
#JG2d | |||
#JG3a(SBB 37) | |||
#JG3b | |||
[[Image:JGgell.jpg]] [[Image:Generuler1kbplus.jpg]] | |||
*notes: SBB37 was too small of a fragment so should have been digested with Eco/BglI not Eco/Bam. | |||
Clotho Entry:<br> | |||
Plate A: A2=JG1A, H1=JG1B <br> | |||
Plate B: A2=JG1B, H1=JG1C <br> | |||
==3/10/10== | |||
To Do: Run analytical gel of miniprep plasmids (JG3A,JG3B)<br> | |||
-[[Template:SBB-Protocols_Enz2 | Eco/BglI Digest]] of miniprep plasmids <br. | |||
-run analytical get of digests, Expected product length (1536/ 684 bp) | |||
Results: | |||
===Jose Gutierrez=== | |||
#ladder | |||
#JG3A | |||
#JG3B | |||
[[Image:JGgel.jpg]] [[Image:Generuler1kbplus.jpg]] | |||
*notes: 3A could have worked, 3B did not work | |||
Clotho Entry:<br> | |||
Plate A: H2=JG3A, | |||
=Team Assay Project= | |||
[[Team 3 Notebook]] | |||
Latest revision as of 02:20, 2 May 2010
Construction Files
SBB30: 5'UTR_rep009
Biobricking of O99 rep Gene
PCR JG001-F and JG002-R on pEC52 (1235 bp, EcoRI/BamHI)
Sub into pBca9523-Bca1144 (EcoRI/BamHI, 910+2472, L)
Product is pBca9523-sbb30 {5'UTR_repO99!}
----------------------------
JG001-F Cloning of O99 rep Gene
ccataGAATTCatgAGATCTaaaaacgattctgacgcattttttatg
JG002-R Cloning of O99 rep Gene
CTGATGGATCCCTACTCTACAAGACCTCGTTTTttc
SBB38: PBad Promoter
Eco/Bam transfer pBjh1601CK-ig114
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-ig114 {AraC-Pbad}
SBB37: PCon Promoter
Eco/Bam transfer pBca1100-Bca1152
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-Bca1152 {Pcon-J23100}
Parts Assembly
2/17/10
Objective: Set up PCR reaction for biobricking of SBB30 009 rep Gene -PCR JG 001-F & JG002-R on pEC52 (1235 bp Eco/Bam)
Procedure:
- convert Oligos to 100uM [X(nmol) + 10*X(ul)]
- dilute stock Oligos to 10uM
- PCR Procedure
2/17/10
Objective: Run Analytical gel on SBB30 (JG01), after confirmation run zymo cleanup
Expected Length (1235 bp)
Results:
Dorothy
- ladder
- 28
- 29
- sbb25
- sbb26
- sbb32
- sbb34
- JG1
- ZHH 1,2 AG
- TN 001/002 PT
- JHA
- JHB
- JHC
PCR product confirmed, continue to Zymo Cleanup
2/24/10
Eco/Bam Digest of SBB30 PCR products
3/1/10
To Do List:
Zymo gell purification of SBB30 Eco/Bam Digest
Eco/Bam transfer pBjh1601CK-ig114
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-ig114 {AraC-Pbad}
Eco/Bam transfer pBca1100-Bca1152
Sub into pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-Bca1152 {Pcon-J23100}
Ligation's:
SBB30 (vector:pBca9523, start: 2:35, end: 3:07)
SBB38 (vector:pBjk2741, start: 3:10, end: 3:40)
SBB37 (vector:pBjk2741, start: 3:10, end: 3:40)
Transformation of Ligation products into competent cells
3/3/10
pick colonies (already done)
- Note: SBB 37 was mostly red colonies on plate only two possible good colonies on plate, probably due to re-ligation of original vector also SBB37 is small which might make Eco/Bam transfer fail, other plates looked good
Mini Prep picked colonies (4 SBB30, 4 SBB38, 2 SBB37) from plates
3/8/10
To Do: Run analytical gel of miniprep plasmids
- Eco/Bam Digest of miniprep plasmids
-run analytical get of digests, Expected product lengths: SBB30(1235 bp); SBB 38 (1253, 2170 bp); SBB37(2170, 50 bp)
Results:
Jose Gutierrez
- JG1a(SBB30)
- JG1b
- JG1c
- JG1d
- JG2a(SBB 38)
- JG2b
- Ladder
- JG2d
- JG3a(SBB 37)
- JG3b
- notes: SBB37 was too small of a fragment so should have been digested with Eco/BglI not Eco/Bam.
Clotho Entry:
Plate A: A2=JG1A, H1=JG1B
Plate B: A2=JG1B, H1=JG1C
3/10/10
To Do: Run analytical gel of miniprep plasmids (JG3A,JG3B)
- Eco/BglI Digest of miniprep plasmids <br.
-run analytical get of digests, Expected product length (1536/ 684 bp)
Results:
Jose Gutierrez
- ladder
- JG3A
- JG3B
- notes: 3A could have worked, 3B did not work
Clotho Entry:
Plate A: H2=JG3A,