SBB10Ntbk-MichelNofal: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
Michel Nofal (talk | contribs) |
Michel Nofal (talk | contribs) (→sbb02) |
||
| Line 397: | Line 397: | ||
===sbb02=== | ===sbb02=== | ||
*Plate was contaminated. (Probably due to contaminated LB) | *Plate was contaminated. (Probably due to contaminated LB) | ||
* | *Mini-Prepped according to [[Template:SBB-Protocols_Micro4 | Macherey-Nagel Miniprep]] protocol: | ||
<pre> | |||
MINIPREP (1mL - 5mL) Procedure for Plasmid DNA Purification | |||
* Pellet the cells (for individual cultures) | |||
1. Pellet around 1.5 mL to 3 mL saturated culture by spinning full speed, 30 seconds. | |||
2. Dump supernatant, repeat to pellet another 1.5 mL (for a total of 3 mL if desired) | |||
* Pellet the cells (for 24-well blocks) | |||
1. In 327, weigh the block on the scale, fill a dirty block with water to the same weight | |||
2. Use the Allegra centrifuge to spin at 5500rpm for 4 minutes | |||
3. Dump supernatant | |||
4. You will need to transfer the cells to microcentrifuge tubes after resuspension in A1 in the alkaline lysis section below | |||
* Do an alkaline lysis | |||
1. Add 250uL of A1 buffer into each tube. Resuspend the cells using a vortexer. | |||
2. Add 250uL of A2 buffer (a base that denatures everything and causes cells to lyse). Gently mix up and down. Solution should become clearer. | |||
3. Add 300uL of A3 buffer (an acid of pH ~5 that causes cell junk - including protein and chromosomal DNA - to precipitate, and leaves plasmids and other small molecules in solution). Slowly invert a few times, then shake. | |||
4. Spin in centrifuge at top speed for 5 minutes. | |||
5. Label columns with an alcohol-resistant lab pen. | |||
* Purify DNA on a column | |||
1. Pour liquid into columns, and place the columns into the centrifuge. Spin at full speed for 15 seconds. | |||
2. Dump liquid out of the collectors under the columns (the DNA should be stuck to the white resin) | |||
3. Wash each column with 500 uL of AW buffer. | |||
4. Spin in centrifuge at full speed for approximately 15 seconds, then flick out the liquid again. | |||
5. Wash with 750uL of A4 buffer (washes the salts off the resins). | |||
6. Spin in centrifuge at full speed for approximately 15 seconds and flick out liquid again. | |||
7. Spin in centrifuge at full speed for 90 sec to dry off all water and ethanol. | |||
8. Label new tubes and put columns in them. | |||
9. Elute them by squirting 50uL of water down the middle of the column (don't let it stick to the sides). | |||
10. Spin in centrifuge at top speed for 30 seconds. | |||
11. Take out columns and cap the tubes. | |||
12. Clean up - note the A1 buffer is stored at 4degC and all the rest at room temperature. | |||
</pre> | |||
===sbb06=== | ===sbb06=== | ||
Revision as of 17:55, 16 March 2010
Construction Files
sbb02: N15 Protelomerase
sbb02: N15 Protelomerase
PCR MNF11-F/MNF12-R on pBca9523-Bca1455 (945 bp, gp = A)
PCR MNF13-F/MNF14-R on pBca9523-Bca1485 (1033 bp, gp = B)
-------------
PCR MNF11-F/MNF14-R on A + B (1925 bp, EcoRI/BamHI)
Sub in pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-sbb02 {<protel!}
-------------
MNF11-F Forward PCR of Part 1 of sbb02 ccagtGAATTCgtccAGATCTAGCAAGGTAAAAATCGGTGAGTTG
MNF12-R SOEing of Part1 of sbb02 ggttacgcttttatcttcactgcgTTTTTTAGCTTGCCCTGAGAAATTAACCG
MNF13-F SOEing of Part2 of sbb02 CGGTTAATTTCTCAGGGCAAGCTAAAAAAcgcagtgaagataaaagcgtaacc
MNF14-R Reverse PCR of Part 2 of sbb02 gcagtggatccTCAGCTGTAGTACGTTTCCCATGC
JCA Notes
- Have gtcc instead of atg in prefix location
- Need to put part on Clotho
- Otherwise correct
- Internal oligos are longer than necessary, but would work
- Due to similarity to other part, some oligos are being replaced:
Construction of sbb02: N15 Protelomerase PCR MNF11-F/CA1472 on Bca1455 1-3 (569bp, gp=A) PCR ca1461/BDBn15001R on Bca1455 1-1 (762bp, gp=B) PCR BDBn15002F/BDBn15002R on Bca1485 2-1 (1023 bp, gp=C) -------------------------------------------------------------- PCR BDBn15001F/BDBn15002R on A+B+C (1924 bp, EcoRI/BamHI) Sub in pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L) Product is pBjk2741-sbb01 {rbs.part!} MNF11-F Forward PCR of Part 1 of sbb02 ccagtGAATTCatgAGATCTAGCAAGGTAAAAATCGGTGAGTTG CA1461 GGAAAGGATTGCAGAAAAGAATAACCGCGAATACTTTTAACGCCTATATGA PCA assembly of protelomerasefiveprime (Bca1455) CA1472 TTGTTGGAATAACTTATAAAGATAGTCACGGCGCTCCTTCCAATCATCACT PCA assembly of protelomerasefiveprime (Bca1455) BDBn15001R Reverse retrieval and SOE-site introduciton on Bca1455 CGCTTTTATCTTCACTGCGTTTTTTAGCTTGCCCTGAG BDBn15002F Forward retrieval and SOE-site introduciton on Bca1485 CTCAGGGCAAGCTAAAAAACGCAGTGAAGATAAAAGCG BDBn15002R Reverse retrieval and BioBricking on Bca1485 CTGATggatccttaGCTGTAGTACGTTTCCCATGCGG part: GATCTAGCAAGGTAAAAATCGGTGAGTTGATCAACACGCTTGTGAATGAGGTAGAGGCCATTGATGCCTCAGACCGCCCACAAGGCGACAAAACGAAGAGAATTAAAGCCGCAGCCGCACGGTATAAGAACGCGTTATTTAATGATAAAAGAAAGTTCCGTGGGAAAGGATTGCAGAAAAGAATAACCGCGAATACTTTTAACGCCTATATGAGCAGGGCAAGAAAGCGGTTTGATGATAAATTACATCATAGCTTTGATAAAAATATTAATAAATTATCGGAAAAGTATCCTCTTTACAGCGAAGAATTATCTTCATGGCTTTCTATGCCTACGGCTAATATTCGCCAGCACATGTCATCGTTACAATCTAAATTGAAAGAAATAATGCCGCTTGCCGAAGAGTTATCAAATGTAAGAATAGGCTCTAAAGGCAGTGATGCAAAAATAGCAAGACTAATAAAAAAATATCCAGATTGGAGTTTTGCTCTTAGTGATTTAAACAGTGATGATTGGAAGGAGCGCCGTGACTATCTTTATAAGTTATTCCAACAAGGCTCTGCGTTGTTAGAAGAACTACACCAGCTCAAGGTCAACCATGAGGTTCTGTACCATCTCCAGCTAAGCCCTGCGGAGCGTACATCTATACAGCAACGATGGGCCGATGTTCTGCGCGAGAAGAAGCGTAATGTTGTGGTTATTGACTACCCAACATACATGCAGTCTATCTATGATATTTTGAATAATCCTGCGACTTTATTTAGTTTAAACACTCGTTCTGGAATGGCACCTTTGGCCTTTGCTCTGGCTGCGGTATCAGGGCGAAGAATGATTGAGATAATGTTTCAGGGTGAATTTGCCGTTTCAGGAAAGTATACGGTTAATTTCTCAGGGCAAGCTAAAAAACGCAGTGAAGATAAAAGCGTAACCAGAACGATTTATACTTTATGCGAAGCAAAATTATTCGTTGAATTATTAACAGAATTGCGTTCTTGCTCTGCTGCATCTGATTTCGATGAGGTTGTTAAAGGATATGGAAAGGATGATACAAGGTCTGAGAACGGCAGGATAAATGCTATTTTAGCAAAAGCATTTAACCCTTGGGTTAAATCATTTTTCGGCGATGACCGTCGTGTTTATAAAGATAGCCGCGCTATTTACGCTCGCATCGCTTATGAGATGTTCTTCCGCGTCGATCCACGGTGGAAAAACGTCGACGAGGATGTGTTCTTCATGGAGATTCTCGGACACGACGATGAGAACACCCAGCTGCACTATAAGCAGTTCAAGCTGGCCAACTTTTCCAGAACCTGGCGACCGGAAGTTGGGGATGAAAACACCAGGCTGGTGGCTCTCCAGAAACTGGACGATGAAATGCCAGGCTTTGCCAGAGGTGACGCTGGCGTCCGTCTCCATGAAACCGTTAAGCAGCTGGTGGAGCAGGACCCATCAGCAAAAATAACCAACAGCACTCTCCGGGCCTTTAAATTTAGCCCGACGATGATTAGCCGGTACCTGGAGTTTGCCGCTGATGCATTGGGGCAGTTCGTTGGCGAGAACGGGCAGTGGCAGCTCAAGATAGAGACACCTGCAATCGTCCTGCCTGATGAAGAATCCGTTGAGACCATCGACGAACCGGATGATGAGTCCCAAGACGACGAGCTGGATGAAGATGAAATTGAGCTCGACGAGGGTGGCGGCGATGAACCAACCGAAGAGGAAGGGCCAGAAGAACATCAGCCAACTGCTCTAAAACCCGTCTTCAAGCCTGCAAAAAATAACGGGGACGGAACGTACAAGATAGAGTTTGAATACGATGGAAAGCATTATGCCTGGTCCGGCCCCGCCGATAGCCCTATGGCCGCAATGCGATCCGCATGGGAAACGTACTACAGCTAAG
sbb06: piggieBac 3'TR
sbb06: piggieBac 3'TR
Pool MNF001 through MNF008, assemble by PCA
PCR MNF01/MNF10 on PCA reaction (267 bp, EcoRI/BamHI)
Sub in pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-sbb06 {<piggieBac-3'TR>}
-------------
MNF01 PCA Assembly of sbb06 CCAGTGAATTCGTCCAGATCTTTTGTTACTTTATAGAAGAAATT
MNF02 PCA Assembly of sbb06 TTATTAAAAAAAAACAAAAACTCAAAATTTCTTCTATAAAGTAA
MNF03 PCA Assembly of sbb06 TTTTGTTTTTTTTTAATAAATAAATAAACATAAATAAATTGTTTG
MNF04 PCA Assembly of sbb06 CACTTACATACTAATAATAAATTCAACAAACAATTTATTTATGTT
MNF05 PCA Assembly of sbb06 TATTATTAGTATGTAAGTGTAAATATAATAAAACTTAATATCTAT
MNF06 PCA Assembly of sbb06 ATATCGAGGTTTATTTATTAATTTGAATAGATATTAAGTTTTATT
MNF07 PCA Assembly of sbb06 AATAAATAAACCTCGATATACAGACCGATAAAACACATGCGTCAA
MNF08 PCA Assembly of sbb06 TACGTTAAAGATAATCATGCGTAAAATTGACGCATGTGTTTTATC
MNF09 PCA Assembly of sbb06 CATGATTATCTTTAACGTACGTCACAATATGATTATCTTTCTAGG
MNF10 PCA Assembly of sbb06 GCAGTGGATCCTTAACCCTAGAAAGATAATCATAT
JCA Notes
- Correct except for prefix sequence should be atg
sbb06: piggieBac 3'TR Pool MNF001 through MNF008, assemble by PCA PCR MNF01/MNF10 on PCA reaction (267 bp, EcoRI/BamHI) Sub in pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L) Product is pBjk2741-sbb06 {<piggieBac-3'TR>} ------------- MNF01 PCA Assembly of sbb06 CCAGTGAATTCatgAGATCTTTTGTTACTTTATAGAAGAAATT MNF02 PCA Assembly of sbb06 TTATTAAAAAAAAACAAAAACTCAAAATTTCTTCTATAAAGTAA MNF03 PCA Assembly of sbb06 TTTTGTTTTTTTTTAATAAATAAATAAACATAAATAAATTGTTTG MNF04 PCA Assembly of sbb06 CACTTACATACTAATAATAAATTCAACAAACAATTTATTTATGTT MNF05 PCA Assembly of sbb06 TATTATTAGTATGTAAGTGTAAATATAATAAAACTTAATATCTAT MNF06 PCA Assembly of sbb06 ATATCGAGGTTTATTTATTAATTTGAATAGATATTAAGTTTTATT MNF07 PCA Assembly of sbb06 AATAAATAAACCTCGATATACAGACCGATAAAACACATGCGTCAA MNF08 PCA Assembly of sbb06 TACGTTAAAGATAATCATGCGTAAAATTGACGCATGTGTTTTATC MNF09 PCA Assembly of sbb06 CATGATTATCTTTAACGTACGTCACAATATGATTATCTTTCTAGG MNF10 PCA Assembly of sbb06 GCAGTGGATCCTTAACCCTAGAAAGATAATCATAT
Part Assembly (Wet Lab)
2/17/2010
sbb02
Note: I'll be sharing oligos with Ben for this part.
- Oligo samples were diluted to 100uM in the tubes they came in, then diluted again to 10uM in separate tubes to be used in the experiment.
- Three PCR reactions were prepared (water added first, protein last) in separate tubes (as described in Cloning by PCR) with these oligos/templates:
- MNF11-F/CA1472 on Bca1455 1-3 (Tube labeled A)
- ca1461/BDBn15001R on Bca1455 1-1 (B)
- BDBn15002F/BDBn15002R on Bca1455 1-2 (C)
24uL ddH2O 3.3uL 10x Expand Buffer "2" 3.3uL dNTPs (2mM in each) 1uL Oligo 1, 10uM 1uL Oligo 2, 10uM 0.5uL Expand polymerase "1" 0.5uL Template DNA
- The predicted products for all three are below 2000kb, so the reaction mixtures should be PCRed up using the 2k55 temperature program.
- The GSIs get to do the actual PCRing.. (aka putting the tubes in the thermocycler and choosing the program, pressing the button, and waiting.)
sbb06
- Completed initial assembly step (as described in PCA Gene Synthesis) in the construction of sbb06 by PCA.
OK, so you've got a bunch of oligos, now what? First, use this recipe and program to do initial assembly of the oligos (do a separate one of these reactions for each chunk you're assembling): Recipe 1. 38 uL ddH2O 2. 5 ul 10x expand buffer 3. 5 ul 2mM dNTPs 4. 1 ul oligo mixture (100uM total, mixture of oligos after combination of 100uM stocks) 5. 0.75 ul Expand polymerase Program (can run JCA/PCA1) 1. 2 min initial denature at 94oC 2. 30 sec denature at 94oC 3. 30 sec anneal at 55oC [This should be the overlap temp of your oligos - vary as needed] 4. 30 sec extension at 72oC 5. repeat 2-4 30 times total
- Again, the GSIs ran the program.
2/22/2010
sbb02
- PCR tubes were retrieved (A, B, and C) after the reactions were completed by the GSIs.
- All three samples were prepared for a preparative gel.
- 6uL PCR product + 4uL loading buffer
- The gel with my samples was run by JHL.
- My PCR products (lanes 10, 11 ,12) appear to be the correct sizes.
- I mistakenly left lab early, and when I came back at 4:30pm, Will and Chris told me that they had already cut the bands out for me and melted them in 650uL ADB buffer (as specified in SOEing PCR).
sbb06
- Small-frag Zymo Cleanup was performed to purify PCA Assembly.
The following procedure removes the polymerase, dNTPs, buffer, and most of the oligonucleotides from a PCR reaction for fragments smaller than 300bp. It also will remove the buffer and restriction enzymes from a restriction digest reaction. 1. Add 100 uL of Zymo ADB buffer (brown bottle) to the reaction. 2. Transfer into the Zymo column (small clear guys) 3. Add 500uL of Ethanol and pipette up and down to mix 4. spin through, discard waste. 5. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 6. spin through, discard waste. 7. Add 200 uL of PE or Zymo Wash buffer 8. spin through, discard waste. 9. spin for 90 seconds, full speed to dry. 10. elute with water into a fresh Eppendorf tube
- The reaction mixture for amplification of the assembled fragments was prepared.
Now, you need to do an amplification of the correct full-length chunks. Clean up the assembly reaction with a zymo column; don't bother running it on a gel - it'll be a smeary mess and won't really help you. Save the purified product in case this step fails! For the amplification reaction, do a normal phusion program with 1 ul of the cleaned up assembly reaction as template, and using the outermost oligos for the chunk. That is: Recipe 1. 1 ul each outer oligo (10 uM) 2. .5 ul phusion 3. 10 ul 5x phusion buffer 4. 5 ul 2mM dNTPs 5. 32.5 ul H2O Program 1. 2 min initial denature at 94oC 2. 30 sec denature at 94oC 3. 30 sec anneal at 60oC [This should be high, as your outer oligos now have a huge overlap with the correct product] 4. 30 sec extension at 68oC 5. repeat 2-4 30 times total
- The program was done by the GSIs.
2/24/2010
sbb02
- PCR product mixture (A+B+C) was purified by Zymo Gel Purification and eluted in 50uL ddH2O:
All spins until the drying step are 15 second full speed spins. 1. cut out bands minimizing extra gel matter. 2. put in ependorf tube and add 600uL of Zymo ADB buffer (brown bottle). 3. heat at 55, shake and/or vortex until the gel has dissolved. 4. If the DNA is <300bp add 250uL of isopropanol 5. transfer into the Zymo column inside a collection tube (small clear guys) 6. spin through, discard waste. 7. Add 200 uL of PE buffer (which is basically 70% ethanol) 8. spin through, discard waste. 9. Add 200 uL of PE buffer 10. spin through, discard waste. 11. spin for 90 seconds, full speed to dry. 12. elute with 8.5 uL of water into a fresh Eppendorf tube
- Second PCR step was set up ( Cloning by PCR):
24uL ddH2O 3.3uL 10x Expand Buffer "2" 3.3uL dNTPs (2mM in each) 1uL Oligo 1, 10uM 1uL Oligo 2, 10uM 0.5uL Expand polymerase "1" 0.5uL Template DNA
- 2K55 program was used again.
sbb06
- Amplification product was retrieved and prepared for an analytical gel.
- 3uL PCR product + 7uL loading buffer
- I ran this gel.
- My amplification product was supposed to be in lane 5. The amplification does not seem to have worked. I'll re-assemble next time..
3/1/2010
sbb02
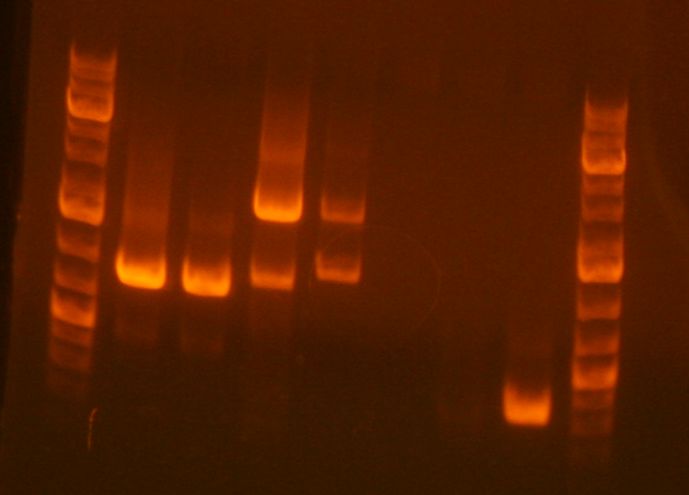
- Prepared PCR product for analytical gel.
- 3uL PCR product + 7 uL loading buffer
- My sample is in the fourth lane. It looks good, although there is another band that looks like it is an incomplete fragment (A+B or B+C).
- Cleaned up the PCR product using a Regular Zymo Cleanup:
The following procedure removes the polymerase, dNTPs, buffer, and most of the oligonucleotides from a PCR reaction. It also will remove the buffer and restriction enzymes from a restriction digest reaction. 1. Add 180 uL of Zymo ADB buffer (brown bottle) to a 33uL or 50uL reaction. 2. Transfer into the Zymo column (small clear guys) 3. spin through, discard waste. 4. Add 200 uL of PE or Zymo Wash buffer (which is basically 70% ethanol) 5. spin through, discard waste. 6. Add 200 uL of PE or Zymo Wash buffer 7. spin through, discard waste. 8. spin for 90 seconds, full speed to dry. 9. elute with water into a fresh Eppendorf tube, use the same volume of water as the volume of the original reaction
sbb06
- Prepared reaction mixture for re-assembly ( PCA Gene Synthesis):
- Labeled 06 Re-As (Re-assembly)
In case the first amplification fails, you can re-amplify using the first amplification as a template to direct the oligos in the second round: Recipe 1. 37 uL ddH2O 2. 5 ul 10x expand buffer 3. 5 ul 2mM dNTPs 4. 1 ul oligo mixture (100uM total, mixture of oligos after combination of 100uM stocks) 5. 1 uL of purified assembly reaction from previous round 6. 0.75 ul Expand polymerase Program 1. 2 min initial denature at 94oC 2. 30 sec denature at 94oC 3. 30 sec anneal at 55oC [This should be the overlap temp of your oligos - vary as needed] 4. 30 sec extension at 72oC 5. repeat 2-4 30 times total Then re-do the amplification step. You can iterate these until you get a full-length product.
- Also re-did the first assembly step from scratch (in case something went wrong along the way).
- Labeled sbb06 S.O. (start over)
- This one runs on the same program as the one above.
OK, so you've got a bunch of oligos, now what? First, use this recipe and program to do initial assembly of the oligos (do a separate one of these reactions for each chunk you're assembling): Recipe 1. 38 uL ddH2O 2. 5 ul 10x expand buffer 3. 5 ul 2mM dNTPs 4. 1 ul oligo mixture (100uM total, mixture of oligos after combination of 100uM stocks) 5. 0.75 ul Expand polymerase
- Both PCR products will be ready next time.
3/3/10
sbb02
- Prepared my PCR product for an Eco/Bam digestion by setting up the following reaction:
8uL of eluted PCR product 1uL of NEB Buffer 2 0.5uL EcoRI 0.5uL BamHI
- Incubated at 37°C for an hour.
- Prepared digest for analytical gel
- 10 uL digest + 1 uL 6x Loading Buffer
- My digest is in lane 4. It looks good. (Better than Ben's in lane 3 at least)
- Cut out the gel and dropped it into an Eppendorf tube.
- Added 600 uL ADB buffer and melted the gel at 55°C
- Performed a Zymo Gel Purification:
* All spins until the drying step are 15 second full speed spins. 1. cut out bands minimizing extra gel matter. 2. put in ependorf tube and add 600uL of Zymo ADB buffer (brown bottle). 3. heat at 55, shake and/or vortex until the gel has dissolved. 4. If the DNA is <300bp add 250uL of isopropanol 5. transfer into the Zymo column inside a collection tube (small clear guys) 6. spin through, discard waste. 7. Add 200 uL of PE buffer (which is basically 70% ethanol) 8. spin through, discard waste. 9. Add 200 uL of PE buffer 10. spin through, discard waste. 11. spin for 90 seconds, full speed to dry. 12. elute with 8.5 uL of water into a fresh Eppendorf tube
sbb06
- Performed a Small-Frag Zymo Cleanup.
- Prepared two identical reactions - one for re-amplification after re-assembly and the other for amplification after starting over:
- I did the second one to get a head start in case re-amplification doesn't work and I did something wrong.
For the amplification reaction, do a normal phusion program with 1 ul of the cleaned up assembly reaction as template, and using the outermost oligos for the chunk. That is: Recipe 1. 1 ul each outer oligo (10 uM) 2. .5 ul phusion 3. 10 ul 5x phusion buffer 4. 5 ul 2mM dNTPs 5. 32.5 ul H2O Program 1. 2 min initial denature at 94oC 2. 30 sec denature at 94oC 3. 30 sec anneal at 60oC [This should be high, as your outer oligos now have a huge overlap with the correct product] 4. 30 sec extension at 68oC 5. repeat 2-4 30 times total
- GSIs ran the program for me on the thermocycler.
3/5/10
sbb06
- Ran analytical gel on re-amplification product.
- 7 uL loading buffer + 3 uL Amp. product
- The re-amplification product is in lane 3. It looks alright, but it's a little faint.
- Performed a Small-frag Zymo Cleanup on the re-amplification product.
3/8/10
sbb02
- Prepared a ligation reaction with the Eco/Bam digest of my sbb02 part and pBjk2741 as the vector.
6.5uL ddH2O 1uL T4 DNA Ligase Buffer (small red or black-striped tubes) 1uL Vector digest 1uL Insert digest 0.5uL T4 DNA Ligase
- Incubated at RT for approximately 30 minutes.
- Put sample on ice and performed heatshock.
1. Thaw a 200 uL aliquot of cells on ice 2. Add 50 uL of water to the cells 3. Add 30 uL of KCM to the cells 4. Put your ligation mixture on ice, let cool a minute or two 5. Add 70 uL of the cell cocktail to the ligation, stir to mix 6. Let sit on ice for 10 min 7. Heat shock for 90 seconds at 42 (longer incubation may work better) 8. Put back on ice for 1 min 9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour 10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight
- Notes on protocol above:
- LB used rather than 2YT in step 9.
- Plated all (~180 uL).
sbb06
- Prepared an Eco/Bam digest of my cleaned up re-amplification product.
8uL of eluted PCR product 1uL of NEB Buffer 2 0.5uL EcoRI 0.5uL BamHI
- Note: when shaking, the cap broke, and I only had 6.8 uL left.
- Incubated at 37°C for approximately an hour.
- Prepared reaction product for digestion analytical gel
- 6.8 uL product + 1 uL 6x loading buffer
- My sbb6 digest is in lane 8 (2nd from the right). It's not perfect, but acceptable.
- Cut out the region of interest, added 600 uL ADB buffer, and melted at 50°C.
- Zymo clean-up next time.
3/10/10
sbb02
- Plate was contaminated. (Probably due to contaminated LB)
- Mini-Prepped according to Macherey-Nagel Miniprep protocol:
MINIPREP (1mL - 5mL) Procedure for Plasmid DNA Purification
* Pellet the cells (for individual cultures)
1. Pellet around 1.5 mL to 3 mL saturated culture by spinning full speed, 30 seconds.
2. Dump supernatant, repeat to pellet another 1.5 mL (for a total of 3 mL if desired)
* Pellet the cells (for 24-well blocks)
1. In 327, weigh the block on the scale, fill a dirty block with water to the same weight
2. Use the Allegra centrifuge to spin at 5500rpm for 4 minutes
3. Dump supernatant
4. You will need to transfer the cells to microcentrifuge tubes after resuspension in A1 in the alkaline lysis section below
* Do an alkaline lysis
1. Add 250uL of A1 buffer into each tube. Resuspend the cells using a vortexer.
2. Add 250uL of A2 buffer (a base that denatures everything and causes cells to lyse). Gently mix up and down. Solution should become clearer.
3. Add 300uL of A3 buffer (an acid of pH ~5 that causes cell junk - including protein and chromosomal DNA - to precipitate, and leaves plasmids and other small molecules in solution). Slowly invert a few times, then shake.
4. Spin in centrifuge at top speed for 5 minutes.
5. Label columns with an alcohol-resistant lab pen.
* Purify DNA on a column
1. Pour liquid into columns, and place the columns into the centrifuge. Spin at full speed for 15 seconds.
2. Dump liquid out of the collectors under the columns (the DNA should be stuck to the white resin)
3. Wash each column with 500 uL of AW buffer.
4. Spin in centrifuge at full speed for approximately 15 seconds, then flick out the liquid again.
5. Wash with 750uL of A4 buffer (washes the salts off the resins).
6. Spin in centrifuge at full speed for approximately 15 seconds and flick out liquid again.
7. Spin in centrifuge at full speed for 90 sec to dry off all water and ethanol.
8. Label new tubes and put columns in them.
9. Elute them by squirting 50uL of water down the middle of the column (don't let it stick to the sides).
10. Spin in centrifuge at top speed for 30 seconds.
11. Take out columns and cap the tubes.
12. Clean up - note the A1 buffer is stored at 4degC and all the rest at room temperature.
sbb06
- Prepared a ligation reaction with the Eco/Bam digest of my sbb06 part and pBjk2741 as the vector.
6.5uL ddH2O 1uL T4 DNA Ligase Buffer (small red or black-striped tubes) 1uL Vector digest 1uL Insert digest 0.5uL T4 DNA Ligase
Note: when shaking, 2.5 out of 10 uL spilled out when cap broke.
- Incubated at RT for approximately 30 minutes.
- Put sample on ice and performed heatshock.
1. Thaw a 200 uL aliquot of cells on ice 2. Add 50 uL of water to the cells 3. Add 30 uL of KCM to the cells 4. Put your ligation mixture on ice, let cool a minute or two 5. Add 70 uL of the cell cocktail to the ligation, stir to mix 6. Let sit on ice for 10 min 7. Heat shock for 90 seconds at 42 (longer incubation may work better) 8. Put back on ice for 1 min 9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour 10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight
- Notes on protocol above:
- LB used rather than 2YT in step 9.
- Plated all (~180 uL).