SBB11Ntbk-Anand Kesavaraju: Difference between revisions
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Welcome to Anand Kesavaraju's 140L Lab Notebook == | ==Welcome to Anand Kesavaraju's 140L Lab Notebook == | ||
__NOTOC__ | |||
==[[User:Anand Kesavaraju]] | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 03 May 2011 (PST)== | ||
Since neither of my parts (sbb1107 & sbb1140) produced viable colonies from the culture tubes, I assisted Jessica Wen with her Analytical Digests (Mapping) protocol: <br> | Presented results/conclusions to class! | ||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 01 May 2011 (PST)== | |||
Continued working on powerpoint, editing notebook - finished both. | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 27 April 2011 (PST)== | |||
Worked on Powerpoint Presentation for Class based on experiment performed. | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 21 April 2011 (PST)== | |||
New graphs from analysis | |||
Viol below: | |||
[[Image:Viol.jpg]] | |||
ToxR below: | |||
[[Image:ToxR.jpg]] | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 13 April 2011 (PST)== | |||
Rishi obtained the data from the Tecan to format and present later. See Rishi's notebook for .csv data files. Curve analysis to be performed later. | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 12 April 2011 (PST)== | |||
On April 11th at 5:30 PM, Jessica started 3mL culutures of ToxR, Violacein, Control | |||
This morning (April 12th 9:30 AM): | |||
@10 AM: pipetted 2mL LB+spec in 39 wells of "block"; add 200uL of cells to each well, 13 wells correspond to 1 type(either ToxR, v or control) | |||
@ 11:15 AM placed blocks in 37C shaker in anderson lab - 1 hour incubation | |||
@ 1:00 PM, Xin Xin and Anand added arabinose to block (see protocol for concentrations added) | |||
Images of blocks shown below: '''Key Observation: Wells 1 and 2 in block 1 were switched while pipetting - note made of this and data were corrected when graphing/performing curve analysis''' | |||
[[Image: Gel_Block_1.jpg]] | |||
[[Image: Gel_Block_2.jpg]] | |||
Added 100uL from each block to tecan (followed Tecan protocol - indicated below) | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 08 April 2011 (PST)== | |||
Group members Rishi, Xin Xin, and Jessica came in early but no one in the Anderson lab was there before 10 AM. Experiment scrapped; to be started over later. | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 06 & 07 April 2011 (PST)== | |||
'''Observations''' | |||
12pm: [[User: Rishi Rawat | Rishi Rawat]] moved cells from incubator to 4C fridge in Anderson lab, next to incubator - some samples suspected to be mislabeled. | |||
All plates had 100s of colonies--> the transformation worked! | |||
However, all of the colonies on the "Violacein" plate were Darkred/purple in color - '''makes sense, Violacein-expressing bacteria are purple in color''' | |||
At ~5PM on April 07th, [[User: Jessica Wen | Jessica Wen]] started overnight cultures for the 3 samples. They are in the 37C shaker in the Anderson Lab | |||
""Overall Protocol"" | |||
List of Materials: | |||
Bac Culture of: | |||
pBCA1256-BCA1144 | |||
1766-BCA1144 | |||
pBCA9523-BCA1144 | |||
ToxR- pBca1256-bdc004-> Plasmid for ToxR named bdc022 | |||
Violacein- pBca9523-jtk2914 | |||
+Spec LB (200mL) | |||
Arabinose x grams | |||
MC1061 | |||
4 Spec Plates | |||
Goal: Evaluate toxicity of ToxR and Violacein(spec) | |||
0.) Do the following procedure once per Toxic gene: | |||
ToxR(Antibiotic = Spectinomycin, plasmid = pBCA1256-BCA1144(RFP) | |||
Violacein(Antibitoic = Spectinomycin, plasmid = pBCA9523-BCA1144(RFP)) | |||
CONTROL: | |||
0.) Prepare a plasmid without the toxic gene- use RFP instead | |||
Overnight: | |||
1.) Transform (heatshock) nontoxic gene plasmids into MC1061, plate +Spec, pick colony, and grow in 2mL liquid media in a 10mL tube in LB + Spec overnight at 37C {O/N Control} | |||
2.) Transform(heatshock) toxic gene plasmids into MC1061,plate +Spec, pick colony, grow in 2mL liquid in a 10mL culture tube in LB +Spec overnight at 37C {O/N Toxic} | |||
1. Thaw a 200 uL aliquot of competent cells on ice | |||
2. Add 50 uL of water to the cells (if greater volume is desired) | |||
3. Add 30 uL of KCM to the cells- did not add KCM, volume cells too small | |||
4. Put your ligation mixture on ice, let cool a minute or two (for Miniprep product, dilute by 10, then use 1uL of dilution)- did not dilute miniprep product | |||
5. Add 70 uL of the cell cocktail to the ligation, stir to mix- added 2uL miniprep plasmid/10uL diluted & 50uL cells | |||
6. Let sit on ice for 10 min- waited 7 min. | |||
7. Heat shock for 90 seconds at 42 (longer incubation may work better) | |||
8. Put back on ice for 1 min | |||
9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour- used LB media | |||
10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight- plate 50uL on Spec plates | |||
Prep: | |||
3.) add 2mL LB + Spec to 10x 10mL test tubes | |||
4.) add 40 uL of O/N Control MC1061 to culture #1a-5a | |||
5.) add 40 uL of O/N Toxic MC1061 to cultures #1b - #5b | |||
6.) grow until OD(600nm) of the cultures is .4 - .6 , on a shaker at 37C | |||
CHECK!!!! IF CUVETTES WTO MEASURE MID LOG PHASEHAVE ENOUGH BACTERIA | |||
Induce: | |||
--make 250uL 20% arabinose solution: | |||
add 50mg Arabinose to ~.25g LB+Spec | |||
--make 2 mL .2% Arabinose solution: | |||
take 20uL of 20% arabinose solution, add to 1880 uL Lb+Spec | |||
--make 2 mL .02% Arabinose solution: | |||
take 200uL of .2% arabinose solution, add to 1800 uL Lb+Spec | |||
6.9) make stock solutions of arabinose. Using LB+SPEC | |||
%Arabin uL(20% or .2% or .02%) to add to 10mL LB+Spec | |||
0.0 0.0 | |||
1.931e-06 .9655 | |||
5.517e-06 2.7585 | |||
1.576e-05 7.88 | |||
4.504e-05 2.252 | |||
0.0001287 6.435 | |||
0.0003677 18.385 | |||
0.00105 52.5 | |||
0.003 1.500 | |||
0.00858 4.290 | |||
0.0245 12.250 | |||
0.07 35.0 | |||
0.2 100.0 | |||
7.) Add Arabinose to a final concentration of: | |||
tube# | |||
1t 0.0 | |||
2t 1.931e-06 | |||
3t 5.517e-06 | |||
4t 1.576e-05 | |||
5t 4.504e-05 | |||
6t 0.0001287 | |||
7t 0.0003677 | |||
8t 0.00105 | |||
9t 0.003 | |||
10t 0.00858 | |||
11t 0.0245 | |||
12t 0.07 | |||
13t 0.2 | |||
→ http://www.pnas.org/content/99/11/7373.full- Arabinose Concentrations | |||
8.) Post induction, Load 100uL from each test tube onto a plate to go into a Tecan plate reader- Do this twice per test tube so that all samples are duplicates. | |||
a.) Setup Tecan to measure OD(600nm) of the each sample in the plate every 15-30 minutes overnight | |||
9.) Copy results from plate reader to powerpoint | |||
10.) average data from duplicate wells | |||
11.) setup a 3D graph time v. [arabinose] v. OD (or alternatively, 5 2D graphs OD v. time) | |||
a.) if the parts are toxic, we expect slower growth and a lower final OD | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 05 April 2011 (PST)== | |||
'''1.) Transform (heatshock) nontoxic gene plasmids into MC1061, plate +Spec'''<br> | |||
'''2.) Transform(heatshock) toxic gene plasmids into MC1061,plate +Spec'''<br> | |||
'''Procedure'''<br> | |||
1. Thaw a 200 uL aliquot of cells on ice<br> | |||
2. Add 50 uL of water to the cells (if greater volume is desired)<br> | |||
3. Add 30 uL of KCM to the cells <br> | |||
4. Put your ligation mixture on ice, let cool a minute or two (for Miniprep product, dilute by 10, then use 1uL of dilution)<br> | |||
5. Add 70 uL of the cell cocktail to the ligation, stir to mix<br> | |||
6. Let sit on ice for 10 min<br> | |||
7. Heat shock for 90 seconds at 42 (longer incubation may work better)<br> | |||
8. Put back on ice for 1 min<br> | |||
9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour<br> | |||
10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight<br> | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 31 March 2011 (PST)== | |||
Finish writing up protocol for Assay project. Part of Group 4: ToxRViol, studying growth curves with ToxR and Violacein with varying concentrations of Arabinose. Growth curves will be studied using simple OD(600nm) measurements with the Tecan. See group page/notebook on OWW Wiki. | |||
Move into this Notebook in future. | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 17 March 2011 (PST)== | |||
Sequencing Analysis! '''Team Joey's Angels''' | |||
Used FinchTV for chromatogram analysis. | |||
Results: [[Image:Sequencing1_Joey's_Angels.xls]] | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 15 March 2011 (PST)== | |||
Assisted [[User: Jessica Wen | Jessica Wen]] with preparing samples for Sequencing. | |||
''Addendum: all toxic class samples were off, so JCA + GSIs are remaking parts and resubmitting'' | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 10 March 2011 (PST)== | |||
[[User: Jessica Wen | Jessica Wen]]'s samples did not work correctly (see 03/08/11 gel), so assisted her again in repeating the Analytical Digests (Mapping) Protocol. | |||
==[[User:Anand Kesavaraju | Anand Kesavaraju]] 08 March 2011 (PST)== | |||
Since neither of my parts (sbb1107 & sbb1140) produced viable colonies from the culture tubes, I assisted [[User:Jessica Wen | Jessica Wen]] with her Analytical Digests (Mapping) protocol: <br> | |||
'''Procedure'''<br> | '''Procedure'''<br> | ||
| Line 21: | Line 227: | ||
Are the calculated sizes consistent with the bands on the gel? | Are the calculated sizes consistent with the bands on the gel? | ||
==[User: Anand Kesavaraju]] 03 March 2011 (PST)== | * After determining which clones are good/bad, place all liquid from two clones into the same positions in racks 'A' & 'B' (i.e. A7 on plate A for clone 1, and A7 on plate B for clone 2) | ||
[[Image:030811Gel.jpg]] | |||
==[[User: Anand Kesavaraju | Anand Kesavaraju]] 03 March 2011 (PST)== | |||
* Colonies were already picked and placed in culture tubes, so today's task was to perform the Miniprep Purification of DNA. | * Colonies were already picked and placed in culture tubes, so today's task was to perform the Miniprep Purification of DNA. | ||
* However, all of the cut-and-paste transformations (e.g. sbb1140: from pBjh1766-Bca1089) failed - Professor Anderson postulates systematically) | * However, all of the cut-and-paste transformations (e.g. sbb1140: from pBjh1766-Bca1089) failed - Professor Anderson postulates systematically) | ||
* In addition, sbb1107 (stress promoter part) had no colony growth in the culture tubes, so both parts failed. | * In addition, sbb1107 (stress promoter part) had no colony growth in the culture tubes, so both parts failed. | ||
* Thus, my task for today was to assist Jessica Wen with her MiniPrep Purification of DNA protocol. <br> | * Thus, my task for today was to assist [[User:Jessica Wen]] with her MiniPrep Purification of DNA protocol. <br> | ||
'''Procedure''' | '''Procedure''' | ||
| Line 55: | Line 265: | ||
*Clean up - note the P1 buffer is stored at 4degC and all the rest at room temperature. <br> | *Clean up - note the P1 buffer is stored at 4degC and all the rest at room temperature. <br> | ||
==[[User:Anand Kesavaraju]] 01 March 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 01 March 2011 (PST)== | ||
'''Ligation'''<br> | '''Ligation'''<br> | ||
| Line 87: | Line 297: | ||
* Plates will be incubated overnight, then colonies will be picked. | * Plates will be incubated overnight, then colonies will be picked. | ||
==[[User:Anand Kesavaraju]] 25 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 25 February 2011 (PST)== | ||
* No class today. | * No class today. | ||
==[[User:Anand Kesavaraju]] 24 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 24 February 2011 (PST)== | ||
'''Zymo Cleanup of Digest'''<br> | '''Zymo Cleanup of Digest'''<br> | ||
| Line 105: | Line 315: | ||
Elute with water into a fresh Eppendorf tube | Elute with water into a fresh Eppendorf tube | ||
==[[User:Anand Kesavaraju]] 22 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 22 February 2011 (PST)== | ||
'''* Eco/Bam digest PCR products protocol today:'''<br> | '''* Eco/Bam digest PCR products protocol today:'''<br> | ||
| Line 121: | Line 331: | ||
* Melt in 600 uL ADB buffer at 55 degrees C. | * Melt in 600 uL ADB buffer at 55 degrees C. | ||
==[[User:Anand Kesavaraju]] 21 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 21 February 2011 (PST)== | ||
* Received email from Professor Anderson: | * Received email from Professor Anderson: | ||
| Line 140: | Line 350: | ||
[[Image:sbb1107AnalyticalGel4.jpg]] | [[Image:sbb1107AnalyticalGel4.jpg]] | ||
==[[User:Anand Kesavaraju]] 18 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 18 February 2011 (PST)== | ||
* Analytical Gel for sbb1140 - see Figure 2 below. No band appeared in lane 5, indicating PCR was unsuccessful.. | * Analytical Gel for sbb1140 - see Figure 2 below. No band appeared in lane 5, indicating PCR was unsuccessful.. | ||
==[[User:Anand Kesavaraju]] 17 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 17 February 2011 (PST)== | ||
* Repeat sbb1140 (BseRImet) PCR - did not work last time. | * Repeat sbb1140 (BseRImet) PCR - did not work last time. | ||
| Line 163: | Line 373: | ||
[[Image:021711-AnalGel2.jpg]] | [[Image:021711-AnalGel2.jpg]] | ||
==[[User:Anand Kesavaraju]] 15 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 15 February 2011 (PST)== | ||
'''PCR of sbb1140 and sbb1107''' | '''PCR of sbb1140 and sbb1107''' | ||
| Line 176: | Line 386: | ||
Followed the following [http://openwetware.org/wiki/Template:SBB-Protocols_PCR2 Protocol for Cloning by PCR]. | Followed the following [http://openwetware.org/wiki/Template:SBB-Protocols_PCR2 Protocol for Cloning by PCR]. | ||
==[[User:Anand Kesavaraju]] 11 February 2011 (PST)== | ==[[User:Anand Kesavaraju | Anand Kesavaraju]] 11 February 2011 (PST)== | ||
I herd u liek notebooks, so I put a notebook in ur notebook so you can look at [http://openwetware.org/wiki/User:Anand_Kesavaraju Anand's Personal Page] | I herd u liek notebooks, so I put a notebook in ur notebook so you can look at [http://openwetware.org/wiki/User:Anand_Kesavaraju Anand's Personal Page] | ||
Latest revision as of 10:42, 3 May 2011
Welcome to Anand Kesavaraju's 140L Lab Notebook
Anand Kesavaraju 03 May 2011 (PST)
Presented results/conclusions to class!
Anand Kesavaraju 01 May 2011 (PST)
Continued working on powerpoint, editing notebook - finished both.
Anand Kesavaraju 27 April 2011 (PST)
Worked on Powerpoint Presentation for Class based on experiment performed.
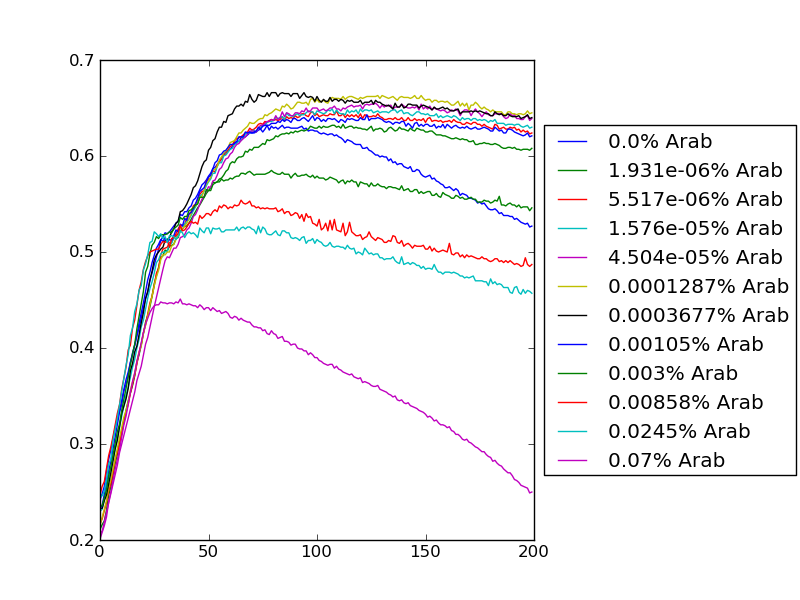
Anand Kesavaraju 21 April 2011 (PST)
New graphs from analysis Viol below:
ToxR below:
Anand Kesavaraju 13 April 2011 (PST)
Rishi obtained the data from the Tecan to format and present later. See Rishi's notebook for .csv data files. Curve analysis to be performed later.
Anand Kesavaraju 12 April 2011 (PST)
On April 11th at 5:30 PM, Jessica started 3mL culutures of ToxR, Violacein, Control
This morning (April 12th 9:30 AM): @10 AM: pipetted 2mL LB+spec in 39 wells of "block"; add 200uL of cells to each well, 13 wells correspond to 1 type(either ToxR, v or control)
@ 11:15 AM placed blocks in 37C shaker in anderson lab - 1 hour incubation
@ 1:00 PM, Xin Xin and Anand added arabinose to block (see protocol for concentrations added)
Images of blocks shown below: Key Observation: Wells 1 and 2 in block 1 were switched while pipetting - note made of this and data were corrected when graphing/performing curve analysis
Added 100uL from each block to tecan (followed Tecan protocol - indicated below)
Anand Kesavaraju 08 April 2011 (PST)
Group members Rishi, Xin Xin, and Jessica came in early but no one in the Anderson lab was there before 10 AM. Experiment scrapped; to be started over later.
Anand Kesavaraju 06 & 07 April 2011 (PST)
Observations 12pm: Rishi Rawat moved cells from incubator to 4C fridge in Anderson lab, next to incubator - some samples suspected to be mislabeled.
All plates had 100s of colonies--> the transformation worked!
However, all of the colonies on the "Violacein" plate were Darkred/purple in color - makes sense, Violacein-expressing bacteria are purple in color
At ~5PM on April 07th, Jessica Wen started overnight cultures for the 3 samples. They are in the 37C shaker in the Anderson Lab
""Overall Protocol""
List of Materials: Bac Culture of: pBCA1256-BCA1144 1766-BCA1144 pBCA9523-BCA1144 ToxR- pBca1256-bdc004-> Plasmid for ToxR named bdc022 Violacein- pBca9523-jtk2914 +Spec LB (200mL) Arabinose x grams MC1061 4 Spec Plates
Goal: Evaluate toxicity of ToxR and Violacein(spec)
0.) Do the following procedure once per Toxic gene:
ToxR(Antibiotic = Spectinomycin, plasmid = pBCA1256-BCA1144(RFP)
Violacein(Antibitoic = Spectinomycin, plasmid = pBCA9523-BCA1144(RFP))
CONTROL: 0.) Prepare a plasmid without the toxic gene- use RFP instead
Overnight: 1.) Transform (heatshock) nontoxic gene plasmids into MC1061, plate +Spec, pick colony, and grow in 2mL liquid media in a 10mL tube in LB + Spec overnight at 37C {O/N Control}
2.) Transform(heatshock) toxic gene plasmids into MC1061,plate +Spec, pick colony, grow in 2mL liquid in a 10mL culture tube in LB +Spec overnight at 37C {O/N Toxic}
1. Thaw a 200 uL aliquot of competent cells on ice
2. Add 50 uL of water to the cells (if greater volume is desired) 3. Add 30 uL of KCM to the cells- did not add KCM, volume cells too small 4. Put your ligation mixture on ice, let cool a minute or two (for Miniprep product, dilute by 10, then use 1uL of dilution)- did not dilute miniprep product 5. Add 70 uL of the cell cocktail to the ligation, stir to mix- added 2uL miniprep plasmid/10uL diluted & 50uL cells 6. Let sit on ice for 10 min- waited 7 min. 7. Heat shock for 90 seconds at 42 (longer incubation may work better) 8. Put back on ice for 1 min 9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour- used LB media
10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight- plate 50uL on Spec plates
Prep:
3.) add 2mL LB + Spec to 10x 10mL test tubes
4.) add 40 uL of O/N Control MC1061 to culture #1a-5a
5.) add 40 uL of O/N Toxic MC1061 to cultures #1b - #5b
6.) grow until OD(600nm) of the cultures is .4 - .6 , on a shaker at 37C
CHECK!!!! IF CUVETTES WTO MEASURE MID LOG PHASEHAVE ENOUGH BACTERIA
Induce:
--make 250uL 20% arabinose solution:
add 50mg Arabinose to ~.25g LB+Spec
--make 2 mL .2% Arabinose solution:
take 20uL of 20% arabinose solution, add to 1880 uL Lb+Spec
--make 2 mL .02% Arabinose solution:
take 200uL of .2% arabinose solution, add to 1800 uL Lb+Spec
6.9) make stock solutions of arabinose. Using LB+SPEC
%Arabin uL(20% or .2% or .02%) to add to 10mL LB+Spec
0.0 0.0
1.931e-06 .9655
5.517e-06 2.7585
1.576e-05 7.88
4.504e-05 2.252
0.0001287 6.435
0.0003677 18.385
0.00105 52.5
0.003 1.500
0.00858 4.290
0.0245 12.250
0.07 35.0
0.2 100.0
7.) Add Arabinose to a final concentration of:
tube#
1t 0.0
2t 1.931e-06
3t 5.517e-06
4t 1.576e-05
5t 4.504e-05
6t 0.0001287
7t 0.0003677
8t 0.00105
9t 0.003
10t 0.00858
11t 0.0245
12t 0.07
13t 0.2
→ http://www.pnas.org/content/99/11/7373.full- Arabinose Concentrations
8.) Post induction, Load 100uL from each test tube onto a plate to go into a Tecan plate reader- Do this twice per test tube so that all samples are duplicates.
a.) Setup Tecan to measure OD(600nm) of the each sample in the plate every 15-30 minutes overnight
9.) Copy results from plate reader to powerpoint 10.) average data from duplicate wells 11.) setup a 3D graph time v. [arabinose] v. OD (or alternatively, 5 2D graphs OD v. time) a.) if the parts are toxic, we expect slower growth and a lower final OD
Anand Kesavaraju 05 April 2011 (PST)
1.) Transform (heatshock) nontoxic gene plasmids into MC1061, plate +Spec
2.) Transform(heatshock) toxic gene plasmids into MC1061,plate +Spec
Procedure
1. Thaw a 200 uL aliquot of cells on ice
2. Add 50 uL of water to the cells (if greater volume is desired)
3. Add 30 uL of KCM to the cells
4. Put your ligation mixture on ice, let cool a minute or two (for Miniprep product, dilute by 10, then use 1uL of dilution)
5. Add 70 uL of the cell cocktail to the ligation, stir to mix
6. Let sit on ice for 10 min
7. Heat shock for 90 seconds at 42 (longer incubation may work better)
8. Put back on ice for 1 min
9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour
10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight
Anand Kesavaraju 31 March 2011 (PST)
Finish writing up protocol for Assay project. Part of Group 4: ToxRViol, studying growth curves with ToxR and Violacein with varying concentrations of Arabinose. Growth curves will be studied using simple OD(600nm) measurements with the Tecan. See group page/notebook on OWW Wiki.
Move into this Notebook in future.
Anand Kesavaraju 17 March 2011 (PST)
Sequencing Analysis! Team Joey's Angels Used FinchTV for chromatogram analysis.
Results: File:Sequencing1 Joey's Angels.xls
Anand Kesavaraju 15 March 2011 (PST)
Assisted Jessica Wen with preparing samples for Sequencing.
Addendum: all toxic class samples were off, so JCA + GSIs are remaking parts and resubmitting
Anand Kesavaraju 10 March 2011 (PST)
Jessica Wen's samples did not work correctly (see 03/08/11 gel), so assisted her again in repeating the Analytical Digests (Mapping) Protocol.
Anand Kesavaraju 08 March 2011 (PST)
Since neither of my parts (sbb1107 & sbb1140) produced viable colonies from the culture tubes, I assisted Jessica Wen with her Analytical Digests (Mapping) protocol:
Procedure
Set up the following 10uL reaction in a PCR tube:
4uL ddH2O 4uL Miniprepped plasmid 1uL 10x NEB Buffer 2 .5uL EcoRI .5uL BamHI (for parts >250bp) or XhoI (for parts <250bp) FOR Righty Methylated Parts use Eco/Xho! Incubate at 37 on the thermocycler for 30 minutes Run an analytical gel Take a picture of the gel
Calculate the expected fragment sizes Are the calculated sizes consistent with the bands on the gel?
- After determining which clones are good/bad, place all liquid from two clones into the same positions in racks 'A' & 'B' (i.e. A7 on plate A for clone 1, and A7 on plate B for clone 2)
Anand Kesavaraju 03 March 2011 (PST)
- Colonies were already picked and placed in culture tubes, so today's task was to perform the Miniprep Purification of DNA.
- However, all of the cut-and-paste transformations (e.g. sbb1140: from pBjh1766-Bca1089) failed - Professor Anderson postulates systematically)
- In addition, sbb1107 (stress promoter part) had no colony growth in the culture tubes, so both parts failed.
- Thus, my task for today was to assist User:Jessica Wen with her MiniPrep Purification of DNA protocol.
Procedure
Miniprep purification of DNA
MINIPREP (2mL) Procedure for Plasmid DNA Purification
(using the QIAGEN QIAPrep Spin Miniprep kit)
!!!!! Make sure Ethanol has been added to the PE Buffer !!!!!
!!!!! Make sure that RNAse has been added to the P1 Buffer !!!!!
*Pellet 2 mL saturated culture by spinning full speed, 30 seconds in a 2mL Microcentrifuge tube. *Dump supernatant *Add 250uL of P1 buffer into each tube. Resuspend the cells thoroughly *Add 250uL of P2 buffer (a base that denatures everything and causes cells to lyse). Gently mix up and down. Solution should become clearer. *Add 350uL of N3 buffer (an acid of pH ~5 that causes cell junk - including protein and chromosomal DNA - to precipitate, and leaves plasmids and other small molecules in solution). Slowly invert a few times, then shake. *Spin in centrifuge at top speed for 5 minutes. *Label blue columns with an alcohol-resistant lab pen. *Pour liquid into columns, and place the columns into the centrifuge. Spin at full speed for 15 seconds. *Dump liquid out of the collectors under the columns (the DNA should be stuck to the white resin) *Wash each column with 500 uL of PB buffer. *Spin in centrifuge at full speed for 15 seconds, then flick out the liquid again. *Wash with 750uL of PE buffer (washes the salts off the resins). *Spin in centrifuge at full speed for 15 seconds and flick out liquid again. *Spin in centrifuge at full speed for 90 sec to dry off all water and ethanol. *Label new Microcentrifuge tubes and put columns in them. *Elute them by adding 50uL of water down the middle of the column (don't let it stick to the sides). *Spin in centrifuge at top speed for 30 seconds. *Take out columns and cap the tubes. *Clean up - note the P1 buffer is stored at 4degC and all the rest at room temperature.
Anand Kesavaraju 01 March 2011 (PST)
Ligation
Procedure
- Set up the following reaction:
6.5uL ddH2O 1uL T4 DNA Ligase Buffer (small red or black-striped tubes) 1uL Vector digest 1uL Insert digest 0.5uL T4 DNA Ligase
- Pound upside down on the bench to mix
- Give it a quick spin to send it back to the bottom of the tube
- incubate on the benchtop for 30min
- Put on ice and proceed to the transformation
Transformation
Procedure
1. Thaw a 200 uL aliquot of cells on ice
2. Add 50 uL of water to the cells (if greater volume is desired)
3. Add 30 uL of KCM to the cells
4. Put your ligation mixture on ice, let cool a minute or two (for Miniprep product, dilute by 10, then use 1uL of dilution)
5. Add 70 uL of the cell cocktail to the ligation, stir to mix
6. Let sit on ice for 10 min
7. Heat shock for 90 seconds at 42 (longer incubation may work better)
8. Put back on ice for 1 min
9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour
10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight
- Plates will be incubated overnight, then colonies will be picked.
Anand Kesavaraju 25 February 2011 (PST)
- No class today.
Anand Kesavaraju 24 February 2011 (PST)
Zymo Cleanup of Digest
Procedure
Transfer into the Zymo column inside a collection tube (small clear guys) Spin through, discard waste. Add 200 uL of A4 Wash Bbuffer (which is basically 70% ethanol) Spin through, discard waste. Add 200 uL of A4 Wash Buffer Spin through, discard waste. Spin for 90 seconds, full speed to dry. Elute with water into a fresh Eppendorf tube
Anand Kesavaraju 22 February 2011 (PST)
* Eco/Bam digest PCR products protocol today:
8uL PCR material 1uL NEB Buffer 2 0.5 uL EcoRI 0.5 uL BamHI
When you do this, you'll run those digests in the thermocycler, so use PCR tubes to set them up. Also, you should NOT add DNA loading buffer before sending your samples upstairs to be run on the gel. It is dangerous to let an active digestion reaction sit with the extra glycerol present in the loading buffer--it can cause degradation of your DNA. You add the loading buffer right before loading the DNA into the gel.
- Extract DNA by cutting out bands corresponding to sbb1107 and sbb1140 lanes in gel (lane 4, lane 5 respectively)
- Melt in 600 uL ADB buffer at 55 degrees C.
Anand Kesavaraju 21 February 2011 (PST)
- Received email from Professor Anderson:
"I noticed on Friday that many of you were using the "AW Wash Buffer" for your Zymo cleanups rather than the "A4 Wash Buffer". Unfortunately, they are both called "Wash Buffer" but they are not the same. AW is equivalent to the Qiagen PB Buffer--it has quanidinium chloride in it. What you wanted was A4, which is ethanol/water. So, your DNA is probably now in the landfill.
I redid all your PCRs and Zymo cleanups and threw out all the old tubes. So, you're back on schedule. I've also modified the protocols and have ordered Qiagen minipreps to eliminate the confusion around the two kits. If you are printing out the protocols, please reprint them before doing things. These are the protocols you will be using:
- http://openwetware.org/wiki/Template:SBB-Protocols_Micro3
- http://openwetware.org/wiki/Template:SBB-Protocols_Zymo1
- http://openwetware.org/wiki/Template:SBB-Protocols_Zymo3
Gel Images:
sbb1140 - lane 7: 3985bp File:Sbb1140AnalyticalGel1.jpg
sbb1107 - lane 3: 1215bp File:Sbb1107AnalyticalGel4.jpg
Anand Kesavaraju 18 February 2011 (PST)
- Analytical Gel for sbb1140 - see Figure 2 below. No band appeared in lane 5, indicating PCR was unsuccessful..
Anand Kesavaraju 17 February 2011 (PST)
- Repeat sbb1140 (BseRImet) PCR - did not work last time.
- Zymo Cleanup (not small fragment) Protocol:
Add 180 uL of Zymo ADB buffer (brown bottle) to a 33uL or 50uL reaction.
Transfer into the Zymo column (small clear guys)
Spin through, discard waste.
Add 200 uL of A4 Wash Buffer (which is basically 70% ethanol)
Spin through, discard waste.
Add 200 uL of A4 Wash Buffer
Spin through, discard waste.
Spin for 90 seconds, full speed to dry.
Elute with water into a fresh Eppendorf tube, use the same volume of water (8μL) as the volume of the original reaction
- Run analytical gel for both (just P_malP for today - see Image below, lane 7)
Anand Kesavaraju 15 February 2011 (PST)
PCR of sbb1140 and sbb1107
Only PCR today
Oligos sbb1140F/R are freeze dried, so rehydrate with appropriate volume of ddH2O and perform a 1:10 dilution to get 10 uM
sbb1107F/R are already at 10 uM
Expand buffer/polymerase for genomic (MG1655 DNA)
Phusion buffer/polymerase for plasmid DNA (pBjh1601CA-Bgl009)
Followed the following Protocol for Cloning by PCR.
Anand Kesavaraju 11 February 2011 (PST)
I herd u liek notebooks, so I put a notebook in ur notebook so you can look at Anand's Personal Page