SBB11Ntbk-RishiRawat: Difference between revisions
Rishi Rawat (talk | contribs) |
Rishi Rawat (talk | contribs) |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
==~~!~~== | ==~~!~~== | ||
==[[User:Rishi Rawat|Rishi Rawat]] 18:30, 1 May 2011 (EDT)== | |||
<nowiki> | |||
#use in python, make sure data file is there too | |||
#!/usr/bin/env python | |||
# encoding: utf-8 | |||
from scipy import linspace, polyval, polyfit, sqrt, stats, randn | |||
from matplotlib.mlab import * | |||
from scipy.optimize import curve_fit | |||
import numpy as np | |||
import matplotlib.pyplot as plt | |||
data = csv2rec('data.csv') | |||
conc = [0.0, | |||
1.9309999999999998e-06, | |||
5.5169999999999999e-06, | |||
1.5760000000000002e-05, | |||
4.5040000000000002e-05, | |||
0.00012870000000000001, | |||
0.00036769999999999999, | |||
0.0010499999999999999, | |||
0.0030000000000000001, | |||
0.0085800000000000008, | |||
0.024500000000000001, | |||
0.070000000000000007, | |||
0.20000000000000001] | |||
#t is in hours | |||
t = [num*6./60 for num in range(200)] | |||
satx = [] | |||
tox_growthrate = [] | |||
viol_growthrate = [] | |||
#prepare plots | |||
left = plt.subplot(1,2,1) | |||
tright = plt.subplot(2,2,2) | |||
bright = plt.subplot(2,2,4) | |||
for i in range(13)[::]: | |||
satx.append(conc[i]) | |||
toxy = np.array(data["tox"+str(i)]) | |||
violy = np.array(data["viol"+str(i)]) | |||
try: | |||
cony = np.array(data["con"+str(i)]) | |||
left.plot(t[0:100],np.log10(cony[0:100]), color='b', label=str(conc[i])) #plot for log OD con v. time | |||
except: pass | |||
left.plot(t[0:100],np.log10(toxy[0:100]), color='r', label=str(conc[i])) #plot for log OD toxR v. time | |||
left.plot(t[0:100],np.log10(violy[0:100]), color= 'g', label=str(conc[i])) #plot for log OD viol v. time | |||
left.set_ylabel("log(OD)") | |||
left.set_xlabel("time(h)") | |||
(tox_ar,tox_br)=polyfit(t[0:20],np.log10(toxy[0:20]),1) | |||
(viol_ar,viol_br)=polyfit(t[0:20],np.log10(violy[0:20]),1) | |||
tox_growthrate.append(tox_ar) | |||
viol_growthrate.append(viol_ar) | |||
#bright.plot(np.log10(satx), tox_growthrate, label = "ToxR") #plot for toxR grothrate v. [arab] | |||
#bright.plot(np.log10(satx), viol_growthrate, label = "Violacein") #plot for viola growthrate v. [arab] | |||
#bright.set_xlabel("log([Arabinose])") | |||
#bright.set_ylabel("growthrate(1/h)") | |||
tox_saturation = [data["tox%i"%(i)][100] for i in range(13)] | |||
viol_saturation = [data["viol%i"%(i)][100] for i in range(13)] | |||
con_saturation = [data["con%i"%(i)][100] for i in range(12)] | |||
#tright.plot(np.log10(satx), tox_saturation, label = 'ToxR') #plot for toxR sat od v. [arab] | |||
#tright.plot(np.log10(satx), viol_saturation, label = 'Viol') #plot for viol sat od v. [arab] | |||
#tright.plot(np.log10(satx)[:-1], con_saturation, label = 'Control') #plot for con sat od v. [arab] | |||
#tright.set_xlabel("log([Arabinose])") | |||
#tright.set_ylabel("OD at saturation") | |||
ax = left | |||
box = ax.get_position() | |||
box = ax.get_position() | |||
ax.set_position([box.x0, box.y0, box.width * 0.7, box.height]) | |||
ax.legend(loc='center left', bbox_to_anchor=(1, 0.5), title="%"+"Arabinose") #legend for left plot | |||
plt.suptitle("Violacein") | |||
plt.show() | |||
</nowiki> | |||
==[[User:Rishi Rawat|Rishi Rawat]] 15:24, 21 April 2011 (EDT)== | |||
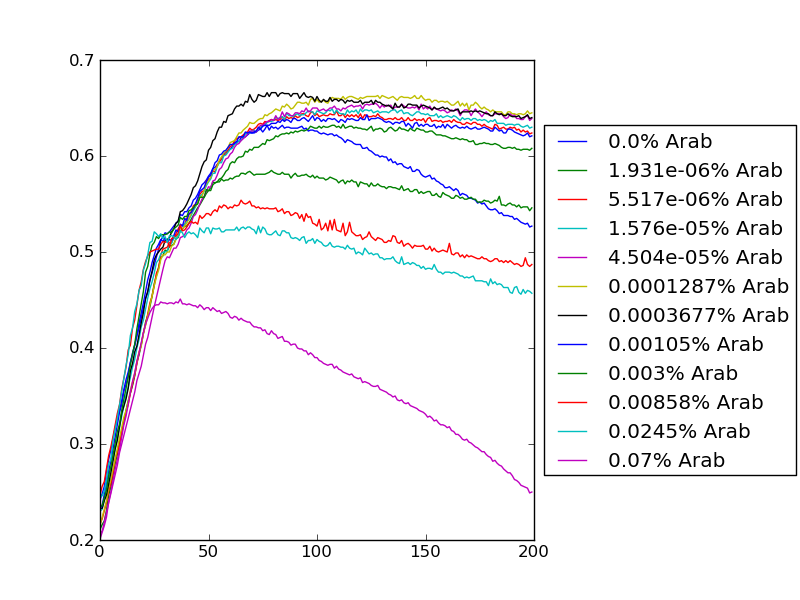
New graphs from analysis | |||
Viol below: | |||
[[Image:Viol.jpg]] | |||
ToxR below: | |||
[[Image:ToxR.jpg]] | |||
==[[User:Rishi Rawat|Rishi Rawat]] 14:00, 13 April 2011 (EDT)== | |||
12pm Rishi gets data from tecan | |||
Rishi formats data and perpares initial graphs | |||
Tecan Output | |||
[[Media:original_data.csv]] | |||
graphing script(use formatted data) | |||
formatted data | |||
[[Media:formattedData.csv]] | |||
==[[User:Rishi Rawat|Rishi Rawat]] 15:00, 12 April 2011 (EDT)== | |||
Yesterday, 530pm, Jessica started 3mL culutures of ToxR, Violacein, Control | |||
This morning: | |||
10AM, pipetted 2mL LB+spec in 39 wells of block | |||
add 200uL of cells to each well, 13 wells correspond to 1 type(either ToxR, v or control) | |||
at 11:15am placed blocks in 37C shaker in anderson lab | |||
TODO: | |||
at 1:00, Xinxin + Anand will add arabinose to block | |||
then they will add 100uL from each block to tecan | |||
==[[User:Rishi Rawat|Rishi Rawat]] 20:44, 8 April 2011 (EDT)== | |||
we came in early but no one in the aderson lab was here before 10. Experiment scrapped. Start over later. | |||
==[[User:Rishi Rawat|Rishi Rawat]] 23:44, 7 April 2011 (EDT)== | ==[[User:Rishi Rawat|Rishi Rawat]] 23:44, 7 April 2011 (EDT)== | ||
Latest revision as of 15:31, 1 May 2011
~~!~~
Rishi Rawat 18:30, 1 May 2011 (EDT)
#use in python, make sure data file is there too #!/usr/bin/env python # encoding: utf-8 from scipy import linspace, polyval, polyfit, sqrt, stats, randn from matplotlib.mlab import * from scipy.optimize import curve_fit import numpy as np import matplotlib.pyplot as plt data = csv2rec('data.csv') conc = [0.0, 1.9309999999999998e-06, 5.5169999999999999e-06, 1.5760000000000002e-05, 4.5040000000000002e-05, 0.00012870000000000001, 0.00036769999999999999, 0.0010499999999999999, 0.0030000000000000001, 0.0085800000000000008, 0.024500000000000001, 0.070000000000000007, 0.20000000000000001] #t is in hours t = [num*6./60 for num in range(200)] satx = [] tox_growthrate = [] viol_growthrate = [] #prepare plots left = plt.subplot(1,2,1) tright = plt.subplot(2,2,2) bright = plt.subplot(2,2,4) for i in range(13)[::]: satx.append(conc[i]) toxy = np.array(data["tox"+str(i)]) violy = np.array(data["viol"+str(i)]) try: cony = np.array(data["con"+str(i)]) left.plot(t[0:100],np.log10(cony[0:100]), color='b', label=str(conc[i])) #plot for log OD con v. time except: pass left.plot(t[0:100],np.log10(toxy[0:100]), color='r', label=str(conc[i])) #plot for log OD toxR v. time left.plot(t[0:100],np.log10(violy[0:100]), color= 'g', label=str(conc[i])) #plot for log OD viol v. time left.set_ylabel("log(OD)") left.set_xlabel("time(h)") (tox_ar,tox_br)=polyfit(t[0:20],np.log10(toxy[0:20]),1) (viol_ar,viol_br)=polyfit(t[0:20],np.log10(violy[0:20]),1) tox_growthrate.append(tox_ar) viol_growthrate.append(viol_ar) #bright.plot(np.log10(satx), tox_growthrate, label = "ToxR") #plot for toxR grothrate v. [arab] #bright.plot(np.log10(satx), viol_growthrate, label = "Violacein") #plot for viola growthrate v. [arab] #bright.set_xlabel("log([Arabinose])") #bright.set_ylabel("growthrate(1/h)") tox_saturation = [data["tox%i"%(i)][100] for i in range(13)] viol_saturation = [data["viol%i"%(i)][100] for i in range(13)] con_saturation = [data["con%i"%(i)][100] for i in range(12)] #tright.plot(np.log10(satx), tox_saturation, label = 'ToxR') #plot for toxR sat od v. [arab] #tright.plot(np.log10(satx), viol_saturation, label = 'Viol') #plot for viol sat od v. [arab] #tright.plot(np.log10(satx)[:-1], con_saturation, label = 'Control') #plot for con sat od v. [arab] #tright.set_xlabel("log([Arabinose])") #tright.set_ylabel("OD at saturation") ax = left box = ax.get_position() box = ax.get_position() ax.set_position([box.x0, box.y0, box.width * 0.7, box.height]) ax.legend(loc='center left', bbox_to_anchor=(1, 0.5), title="%"+"Arabinose") #legend for left plot plt.suptitle("Violacein") plt.show()
Rishi Rawat 15:24, 21 April 2011 (EDT)
New graphs from analysis Viol below:
ToxR below:
Rishi Rawat 14:00, 13 April 2011 (EDT)
12pm Rishi gets data from tecan
Rishi formats data and perpares initial graphs
Tecan Output Media:original_data.csv
graphing script(use formatted data)
formatted data Media:formattedData.csv
Rishi Rawat 15:00, 12 April 2011 (EDT)
Yesterday, 530pm, Jessica started 3mL culutures of ToxR, Violacein, Control
This morning: 10AM, pipetted 2mL LB+spec in 39 wells of block
add 200uL of cells to each well, 13 wells correspond to 1 type(either ToxR, v or control)
at 11:15am placed blocks in 37C shaker in anderson lab
TODO:
at 1:00, Xinxin + Anand will add arabinose to block
then they will add 100uL from each block to tecan
Rishi Rawat 20:44, 8 April 2011 (EDT)
we came in early but no one in the aderson lab was here before 10. Experiment scrapped. Start over later.
Rishi Rawat 23:44, 7 April 2011 (EDT)
As far as sample mislabeling goes, everything may be in order. Xinxin suggested that the purple colonies may be normal b/c Violacein is a chromophore and makes cells look purple. That suggests that nothing was mislabeled. So we proceded.
At ~5PM today, Jessica started O/N cultures for the 3 samples. They are in the 37C shaker in the anderson Lab(shaker(not incubator)--I hope).
Plan for FRIDAY and SATURDAY:
Rishi, Jessica, Xinxin will meet at the Anderson lab at 8am. They will start 41 cultures(2mL each) in "blocks"(we will ask Anderson Lab students to show these to us) with 40uL of O/N cultures. Each of the 3 samples gets 13 tubes; the 2 extra tubes will be used to determine OD. These cultures will grow at 37C in the warm room while we prepare 3 stock solutions of Arabinose.
Approx 2-3 hours later(target 11am) when the OD is high enough(based on Spectrophotometer readings), the arabinose will be used to induce the cultures. 100uL of each culture will go into a single well in the Tecan Plate(and we will do this 2x per culture to get duplicates). The run will go overnight.
Saturday, Anand will get the data from the Tecan reader and shut it off. We will discuss this with JCA/TAs after lecture tomorrow.
Rishi Rawat 12:00, 6 April 2011 (EST)
12pm: Rishi Moves cells from incubator to 4C fridge in Anderson lab, next to incubator
I suspect some samples may be mislabeled.
All plates had 100s of colonies--> the transformation worked
However,all of the colonies on the "Violacein" plate were Darkred/purple in color And all of the colonies on the control(pBCA1766) were white. This is backwards. The control should be the only plasmid with RFP. So perhaps these samples got switched.
Rishi Rawat 11:20, 5 April 2011 (EST)
1.) Transform (heatshock) nontoxic gene plasmids pBCA1766 into MC1061, plate +Spec 2.) Transform(heatshock) toxic gene plasmids into MC1061,plate +Spec Procedure
1. Thaw cells on ice
2. add 2uL DNA to each HShock tube 3. add 50uL cells to each tube 3.5 sit on ice 10 min 4. shock 90sec 42C 5. place on ice 5 min 6. add 200uL LB 7. place on shaker in Anderson lab at 37C for 55 min
Anand + Xinxin will do: 8. plate on +spec plates 9. leave in 37C incubator o/n
Rishi Rawat 13:22, 10 March 2011 (EST)
--Submitted samples for sequencing
--Used samples:
1118-1,1118-2
1102-1,1102-2
1122-1,1122-2
-Done
Rishi Rawat 12:25, 8 March 2011 (EST)
--Nothing worked today. --I was supposed to run an analytical gel and then choose samples to submit for sequencing. --Analytical gel was faint. could not see insert bands, only vector bands --Did not have time to submit stuff for sequencing
Rishi Rawat 12:45, 3 March 2011 (EST)
--Colonies from transformation look good. 100+ on each plate. Similar sized colonies.
--GSIs picked and grew colonies in 4mL LB + Kan(4 samples per plate)
--GOAL: do minipreps -->used 2mL of microbe cultures to do Quigen minipreps -->Followed Qiagen protocol EXACTLY --> eluted in 50uL ddH2O, total of 12 tubes
Rishi Rawat 12:04, 1 March 2011 (EST)
Goal: do Ligations + Transformations + Plating
for RR1102, RR1119, RR1122:
--used 1uL insert
--1ul Vector(pBCA1601KC)
--.5uL t4 ligase
--1uL buffer
--6.5uL water
--> Ran ligation 40min at room temp
Transformation:
Thawed Lefty Cells
--Added 30uL KMC to a 200uL aliquot
--Added 70uL of mixture to each transformation reaction(I may have pipetted cells up/down too much-> lower tf efficiency). I used the total ligation mixture
--let sit on ice ~10min
--heatshock 90sec at 43C
--cool on ice ~5min
--plate total mixture on Kan+ plates
Rishi Rawat 14:04, 24 February 2011 (EST)
Goal: do Zymo gel extraction cleanup(to protocol) for the solubulized gels from 2-22-10, corresponding to SBB1102, 1118, 1122
---Accessed solubulized gel samples
---Added to Zymo Column + Spin
---[ + 200uL A4(Wash Buffer) + spin](x2)
---spin 90sec
---elute 9uL ddH20
Samples are ~called [RR1102, RR1119, RR1122] RE Digest, Zymo, Insert
Next Steps:
Ligation with Vector(~1hr)
and Transformation by Heat Shock(~10min + 1hr recovery)
and Plating(~30min)
Rishi Rawat 13:50, 22 February 2011 (EST)
CA redid all of the PCRs(for gels see post: "JCAnderson 19:32, 21 February 2011 (EST)" at http://openwetware.org/wiki/SBB11_gels).
Then he did PCR cleanups for each rxn.
I am starting the day with SB1118, SB1122, SB1102.
goal: do digestions.
Protocol: 8ul pcr product, 1 uL NEB2, .5uL EcoRI, .5uL BamHI
-->Mixed materials
-->Ran Prep. Gel(http://openwetware.org/wiki/Image:022211-PrepGel4.jpg) with complete digestion mixture(10ul) + 1uL loading Dye.
---The last three lanes correspond to 1102, 1118, 1122(in order from L to R)
---the main bands of all three samples are approx. the right size
---1118 did not work as well as 1102 or 1122.
---1102 displays multiple bands.
--> extracted the main band for each sample
--> added 600uL buffer(yellow), in brown tube, to bring gel into solution; placed at 55C for approx 7 min + inversions
--> stored in box, probably to be kept at -20C - 4C
Rishi Rawat 16:29, 18 February 2011 (EST)
Ran analytical gel:
Lanes 6,7,8 represent PCRs 2, 1, 3. All three PCRs Worked. but pcr 1 was weak.
http://openwetware.org/wiki/Image:021811-AnalGel1.jpg I expected 1kb, ~380 bp, 1kb. The band at ~380 was too faint.
I need to redo the PCR: cycle 2k45 with + DMSO(number 5) and -DMSO(number4)
I also did pcr cleanups for 1,2,3:
PCR cleanup:
180 ADB
Zymo Column -> spin
200ul Zymo Wash(x2)
90 sec spin
elute 30ul
These pcr products are stored.
Rishi Rawat 14:05, 17 February 2011 (EST)
NEXT STEPS:
Day1:
1.) Analytical gel
Run: 2(~1kb), 1(~300bp), 3(~1kb)
2.) (20Min) PCR cleanup; Regular Zymo Cleanup(frag>200bp) http://openwetware.org/wiki/Template:SBB-Protocols_Zymo1; elute in 33uL
3.) (1hr) RE Digest Eco/Bam; http://openwetware.org/wiki/Template:SBB-Protocols_Enz2
4.) Run Preparative Gel
Day2:
4.5) (30min) Gel purify; http://openwetware.org/wiki/Template:SBB-Protocols_Zymo3
5.) (30min)Ligate; http://openwetware.org/wiki/Template:SBB-Protocols_Enz4
6.) (1hr)transform; http://openwetware.org/wiki/Template:SBB-Protocols_Micro1
7.) colony stuff...
Rishi Rawat 12:58, 17 February 2011 (EST)
I messed up the PCR. I used 2.4uL H20 instead of 24uL. MUST REDO PCR.
The CORRECT mixture:
24uL ddH2O
3.3uL 10x Expand Buffer "2"
3.3uL dNTPs (2mM in each)
1uL Oligo 1, 10uM
1uL Oligo 2, 10uM
0.5uL Expand polymerase "1"
0.5uL Template DNA
Made Master Mix(4 reactions):
96 H20
13.2 Buffer 2
13.2 NTP
---> use 30.6uL per pcr tube
Rxns:add to the 30.6uL
1.)1
.5 MG1655
1 1118F
1 43R
.5 Pol
2.)
.5 MG1655
1 48F
1 48R
.5 Pol
3.)
.5 MG1655
1 27F
1 27R
.5 Pol
- ////not sure how much pol actually got used in this reaction. Perhaps less than 1uL, perhaps a bit more.
Rishi Rawat 02:03, 15 February 2011 (EST)
Recieved oligo RRsb1118Forward, resuspended in water(added 295 uL ddH2O) to make 100uM stock.
Did PCR for sb1118, 1122, 1102 used mg1655 template
sb1118 F: sb1118F R: ss43R goal:371bp
sb1122: F: ss48F R: ss48R goal:1010bp
sb1102: F: ss27F R: ss27R goal:986bp
used extend protocol:
2.4uL h20
3.3uL 10x expand2
3.3uL dNTP
1uL oligo
1uL oligo
.5uL expand
.5uL Template
Placed reaction tubes in <2k bp PCR Reaction
Rishi Rawat 02:03, 9 February 2011 (EST)
Hello!
Rishi Rawat 02:02, 9 February 2011 (EST)
This is my first entry!