Sacks:RAD-seq: Difference between revisions
(→References and additional reading: basics sites) |
|||
| (27 intermediate revisions by the same user not shown) | |||
| Line 14: | Line 14: | ||
** ATP | ** ATP | ||
** Phusion High Fidelity PCR master mix | ** Phusion High Fidelity PCR master mix | ||
* 100 bp DNA ladder | |||
* gel loading dye that does NOT have bromophenol blue | |||
Note: ''Msp''I is not a heat-inactivated enzyme, but I have found that the protocol works anyway. Between the ligation and gel extraction steps, I keep the sample on ice to prevent any residual digestion activity. | Note: ''Msp''I is not a heat-inactivated enzyme, but I have found that the protocol works anyway. Between the ligation and gel extraction steps, I keep the sample on ice to prevent any residual digestion activity. | ||
| Line 47: | Line 49: | ||
* Ordinary PCR machine | * Ordinary PCR machine | ||
* Agarose gel rig | * Agarose gel rig | ||
* UV transilluminator for gel excision | |||
* Bioanalyzer | * Bioanalyzer | ||
* real-time PCR machine (we just pay the core facility to do that part) | * real-time PCR machine (we just pay the core facility to do that part) | ||
| Line 99: | Line 102: | ||
In most cases, the concentration estimate via Picogreen should be lower than the concentration estimate via Nanodrop. This is because Nanodrop measures DNA + RNA, whereas Picogreen only measures DNA. Why didn’t we just use Picogreen to begin with? Because it can measure a much narrower range of concentrations than Nanodrop can. If the standard curve were any more concentrated, it would not be linear. | In most cases, the concentration estimate via Picogreen should be lower than the concentration estimate via Nanodrop. This is because Nanodrop measures DNA + RNA, whereas Picogreen only measures DNA. Why didn’t we just use Picogreen to begin with? Because it can measure a much narrower range of concentrations than Nanodrop can. If the standard curve were any more concentrated, it would not be linear. | ||
Based on the Picogreen concentration estimates, dilute the DNA to 50 ng/μL in 10 mM Tris (and 0.1 mM EDTA, optional). | '''Based on the Picogreen concentration estimates, dilute the DNA to 50 ng/μL in 10 mM Tris (and 0.1 mM EDTA, optional).''' | ||
Notes for samples of concentration lower than 50 ng/μL: | |||
* If you have a lot of samples that are '''30-50 ng/μL''', you can dilute all samples for your library to 30 ng/μL or 40 ng/μL instead of 50. The amount of adapter that you add at the ligation step (see below) should be reduced proportionately. | |||
* For samples in the '''10-50 ng/μL''' range, a cheap and efficient way to concentrate them is by [[Purification of DNA | isopropanol precipitation]]: | |||
** Combine 200 μL DNA sample, 20 μL 3M sodium acetate, and 200 μL isopropanol. | |||
** Mix well by inversion. Place in the freezer for at least an hour. | |||
** Spin down 10 minutes in the centrifuge. | |||
** Pour off the liquid, taking care to keep the pellet. | |||
** Add 200 μL 70% ethanol to rinse. Invert a few times. | |||
** Spin down 1 minute, then pour off the ethanol, again being careful not to lose the pellet. | |||
** Allow to dry on the lab bench. | |||
** Resuspend the DNA in 20 μL TE. | |||
** Requantify with Picogreen, then dilute to 50 ng/μL. | |||
===Restriction digestion and ligation=== | ===Restriction digestion and ligation=== | ||
Restriction digestion master mix: | |||
{| border="1" | |||
! Ingredient !! For one sample !! For one plate | |||
|- | |||
| 50 ng/ul DNA || 5 ul || - | |||
|- | |||
| 10X NEBuffer 4 || 1.5 ul || 165 ul | |||
|- | |||
| PstI-HF, 20,000 U/mL || 0.25 ul || 27.5 ul | |||
|- | |||
| MspI, 20,000 U/mL || 0.25 ul || 27.5 ul | |||
|- | |||
| Nuclease-free water || 8 ul || 880 ul | |||
|} | |||
(I have also used DNA at a concentration of 100 ng/ul because that was what Keck wanted for GoldenGate, so then I used 2.5 ul DNA and 10.5 ul water.) | |||
Do this in a 96-well plate. Pipette the DNA into the wells and then add 10 ul of master mix to everything. Pick one well that will not have DNA in it. This will be an important control later on to demonstrate that this library was not contaminated with another library (which will have a different empty well). | |||
Run the Digest program on the PCR machine: 3 hours at 37°C, then 20 minutes at 80°C. | |||
Using a multichannel pipette, add 1.5 μL of 0.1 μM PstI adapters to their corresponding wells on the digestion plate. (Do add the adapter corresponding to the well that has no DNA in it.) | |||
Ligation master mix, keep on ice until use: | |||
{| border="1" | |||
! Ingredient !! For one sample !! For one plate | |||
|- | |||
| 10X Ligase buffer with ATP || 1 ul || 110 ul | |||
|- | |||
| 10 μM MspI adapter || 0.5 ul || 55 ul | |||
|- | |||
| 10 mM ATP || 1.5 ul || 165 ul | |||
|- | |||
| T4 Ligase, 2M U/mL || 0.1 ul || 11 ul | |||
|- | |||
| Nuclease-free water || 5.4 ul || 594 ul | |||
|} | |||
Add 8.5 μL of ligation master mix to each well of the digestion plate. | |||
Run on the "ligate" program on the PCR machine: 2 hours at 25°C, 20 minutes at 65°C. | |||
===Cleanup and amplification=== | ===Cleanup and amplification=== | ||
* Using a multichannel pipette and a PCR 8-well strip tube, pool all the columns together, adding 5 μL from each well of the plate to the wells on the strip tube. | |||
* Pipette the 60 μL out of each well on the strip tube into one 1.5 mL tube. Mix well so that all samples are combined evenly. Freeze or keep on ice. | |||
* Pour a 2% agarose gel with ethidium bromide. Make it nice and deep; my recipe is 3 g agarose, 150 mL 1X TAE, and 7.5 μL ethidium bromide solution. Use a wide-toothed comb. | |||
* Take 40 μL (or more depending on your well volume) of your pooled library and combine it with a loading dye that does not have bromophenol blue. I actually use 10 μL of Promega GoTaq Green PCR buffer, despite the fact that I'm not doing PCR, since it doubles as a loading dye and lacks bromophenol blue. | |||
* I recommend cleaning out your gel rig and putting in fresh TAE, since you especially want to avoid any contamination from other Illumina libraries. | |||
* Run your ~50 μL of library plus loading dye on the gel. The lane with the library should have a lane of 100 bp ladder on either side of it. You can put multiple libraries on one gel, but leave several empty lanes between them. | |||
* The gel doesn't need to be run very long. I would go 20 minutes at 100 V, or until the ladder bands below 500 bp are distinguishable. | |||
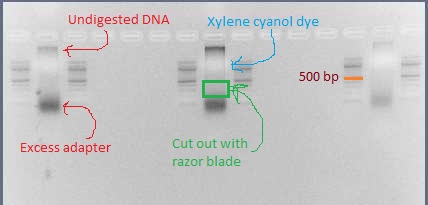
* The library should look like a smear. There may be some undigested DNA (a band in the 10's of kb) but that is okay as long as most of the DNA is digested. There may also be a thick band of leftover adapter below 100 bp. | |||
* Using a clean razor blade for each library, cut out the smear between 200 bp and 500 bp. There should definitely be DNA visible in this range. | |||
[[Image:LVCpooledLibraryGel.jpg|Three pooled ligations ready for gel extraction]] | |||
* Use the Qiagen gel extraction kit to purify the DNA out of this gel slice. Elute in the lower volume (30 μL EB). | |||
* Run the Illumina PCR: | |||
** 3 μL gel-extracted library | |||
** 2 μL 10 μM forward + reverse Illumina primers (PCR1 and PCR2) | |||
** 25 μL 2X Phusion Master mix | |||
** 20 μL nuclease-free water | |||
* PCR program: | |||
** 98°C 30 seconds | |||
** 15 cycles of 98°C 10 seconds, 65°C 30 seconds, 72°C 30 seconds | |||
** 72°C 5 minutes | |||
* Run 5 μL of the PCR product out on a 2% agarose gel. Look to see whether there is primer-dimer visible. | |||
[[Image:LVCPCRLibraryGel.jpg|Nine libraries post-PCR. A second gel (with space in between libraries) will be needed for extraction of the libraries, to eliminate the primer-dimer.]] | |||
* If there is primer-dimer visible, run the remaining 45 μL of PCR product on a 2% agarose gel and extract the library (as was done pre-PCR). Follow the instructions in the Qiagen gel extraction kit as specified for sequencing. (After binding DNA to the column, do a wash with QG. When rinsing with PE, let sit for 2-5 minutes before spinning.) | |||
* If there is no primer-dimer visible, use the Qiagen PCR cleanup kit to purify the remaining 45 μL of PCR product. | |||
===Quality control=== | ===Quality control=== | ||
* Quantify the purified PCR product using the Picogreen protocol as above. Expected concentrations are in the 10's of ng/μL. | |||
* Run on a DNA 1000 chip on the Bioanalyzer. There should be a smooth curve from around 200 to 500 bp. Any sharp peaks could indicate that the enzymes were cutting in a repetitive region of the genome, in which case it is best to choose different enzymes. Use the Bioanalyzer software to calculate the average fragment size. | |||
** If there is primer-dimer remaining in the library, it will be visible as a sharp peak at a lower molecular weight than the broad peak for the library. (The library pictured below does not have primer-dimer.) | |||
[[Image:LVCbioanalyzerExample.jpg | Expected bioanalyzer results on RADseq libraries using this protocol]] | |||
* Calculate the concentration of the PCR product in nM. Keck supplies a worksheet for this calculation. If <math>x</math> is the concentration in ng/μL, <math>y</math> is the average size in base pairs, and <math>z</math> is the concentration in nM, then <math>z = \frac{10^6*x}{649y}</math>. | |||
* Dilute the purified PCR product to 10 nM in EB (10 mM Tris). | |||
* Give 20 μL of 10 nM library to the core facility (Keck). They will use real-time PCR to confirm a concentration of 10 nM. Using Illumina Hi-Seq, do one lane of 100 bp single-end reads. | |||
==Bioinformatics== | ==Bioinformatics== | ||
We are aligning ''Miscanthus'' sequences to the ''Sorghum'' genome, which can be downloaded at [http://www.phytozome.net/sorghum phytosome.net]. | |||
We are primarily using the software package [http://creskolab.uoregon.edu/stacks/ Stacks] for processing the data. | |||
Computation is done on [http://help.igb.uiuc.edu/Biocluster Biocluster], a Unix cluster at the University of Illinois. | |||
Example script for initial processing of the data: [[Media:LVC121113preprocess.txt]] | |||
==Notes== | ==Notes== | ||
| Line 117: | Line 214: | ||
Please sign your name to your note by adding <font face="courier"><nowiki>'''*~~~~''':</nowiki></font> to the beginning of your tip. | Please sign your name to your note by adding <font face="courier"><nowiki>'''*~~~~''':</nowiki></font> to the beginning of your tip. | ||
==References== | ==References and additional reading== | ||
* Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, and Mitchell SE (2011) | * Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, et al. (2008) Rapid SNP Discovery and Genetic Mapping Using Sequenced RAD Markers. PLoS ONE 3(10): e3376. [[doi:10.1371/journal.pone.0003376]] | ||
* Catchen JM, Amores A, Hohenlohe P, Cresko W, and Postlethwait JH (2011) Stacks: building and genotyping loci de novo from short-read sequences. G3: Genes, Genomes, Genetics 1:171-182. [[doi: 10.1534/g3.111.000240]] | |||
* Davey JL and Blaxter MW (2010) RADSeq: next-generation population genetics. Briefings in Functional Genomics 9(5):416-423. [[doi:10.1093/bfgp/elq031]] | |||
* Davey, J. W., Cezard, T., Fuentes-Utrilla, P., Eland, C., Gharbi, K. and Blaxter, M. L. (2012), Special features of RAD Sequencing data: implications for genotyping. Molecular Ecology. [[doi: 10.1111/mec.12084]] | |||
* Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, and Mitchell SE (2011) A robust, simple Genotyping-by-Sequencing (GBS) approach for high diversity species. PLoS One 6(5): e19379. [[doi:10.1371/journal.pone.0019379]] | |||
* Hohenlohe PA, Catchen J, Cresko WA (2012) Population Genomic Analysis of Model and Nonmodel Organisms Using Sequenced RAD Tags. In: Data Production and Analysis in Population Genomics, Pompanon F and Bonin A, eds. 235-260. [[doi:10.1007/978-1-61779-870-2_14]] | |||
* Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double Digest RADseq: An Inexpensive Method for De Novo SNP Discovery and Genotyping in Model and Non-Model Species. PLoS ONE 7(5): e37135. [[doi:10.1371/journal.pone.0037135]] | |||
* Serang O, Mollinari M, Garcia AAF (2012) Efficient Exact Maximum a Posteriori Computation for Bayesian SNP Genotyping in Polyploids. PLoS ONE 7(2): e30906. [[doi:10.1371/journal.pone.0030906]] | |||
===The basics=== | |||
* An overview of restriction digestion and ligation: [http://www.vivo.colostate.edu/hbooks/genetics/biotech/enzymes/index.html] | |||
* [[DNA ligation]] | |||
* [[Restriction digest]] | |||
==Contact== | ==Contact== | ||
* | * [[User:Lindsay V. Clark]] | ||
or instead, [[Talk:{{PAGENAME}}|discuss this protocol]]. | or instead, [[Talk:{{PAGENAME}}|discuss this protocol]]. | ||
Revision as of 13:13, 21 January 2013
Overview
This is a protocol for generating RAD libraries for Illumina sequencing. With this technique, 96 samples can be multiplexed into one sequencing library, and only tags adjacent to PstI sites are sequenced. This is a cheap way to both mine and genotype large numbers of SNPs. This is the protocol developed in Erik Sacks' lab at UIUC by Lindsay Clark, based on protocols from Pat Brown and Megan Hall.
Materials
Reagents
- Quant-iT Picogreen kit (Invitrogen)
- Qiagen gel purification kit
- Qiagen PCR cleanup kit
- From New England Biolabs:
- PstI-HF, 20,000 U/mL
- MspI, 20,000 U/mL
- T4 DNA ligase, 2,000,000 U/mL
- ATP
- Phusion High Fidelity PCR master mix
- 100 bp DNA ladder
- gel loading dye that does NOT have bromophenol blue
Note: MspI is not a heat-inactivated enzyme, but I have found that the protocol works anyway. Between the ligation and gel extraction steps, I keep the sample on ice to prevent any residual digestion activity.
- You will also need a black microtiter plate for the Picogreen assay.
Oligonucleotides
PstI adapters
This is the most expensive part of the protocol other than the sequencing itself, since 192 oligonucleotides must be ordered.
Adapter 1 top: 5'GATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTxxxxTGCA3'
Adapter 1 bottom: 5'yyyyAGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTAGATC3'
Where xxxx and yyyy are the barcode and its reverse complement, respectively.
Barcodes and oligo sequences are from Pat Brown's lab.
Other oligos
MspI adapters:
- A2top:
5'CGCTCAGGCATCACTCGATTCCTCCGAGAACAA3' - A2bot:
5'CAAGCAGAAGACGGCATACGACGGAGGAATCGAGTGATGCCTGAG3'
Illumina PCR primers:
- PCR1:
5'AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT3' - PCR2:
5'CAAGCAGAAGACGGCATACGA3'
Equipment
- Nanodrop spectrophotometer
- BioTek Synergy plate reader (for reading fluorescence)
- Ordinary PCR machine
- Agarose gel rig
- UV transilluminator for gel excision
- Bioanalyzer
- real-time PCR machine (we just pay the core facility to do that part)
Procedure
Adapter prep
Top and bottom strands of adapters need to be annealed 1X Annealing Buffer, which is 10 mM Tris, 50 mM NaCl.
The annealing program is:
- 95°C 5 minutes
- Ramp down -0.1°C every 2 seconds (or -1°C every 20 seconds) to 25°C.
My protocol:
- Pat Brown provided us with a plate of PstI adapters that are at 1 μM. I took a bottle of autoclaved 1X Annealing Buffer, added 45 μl to each well of a 96-well plate, then transferred 5 μl from the 1 μM plate to make a 0.1 μM working stock.
- MspI adapters are ordered like normal oligos, and I have 100 μM concentrated stocks in TE. To make a 10 μM stock:
- 20 μl A2top, 100 μM
- 20 μl A2bot, 100 μM
- 20 μl 500 mM NaCl
- 2 μl 1M Tris
- 138 μl nuclease-free water
- Mix well, add 100 μl to each of two PCR tubes, and run them on the annealing program ("Adapt" on the PCR machine).
DNA quantification and dilution
Nanodrop first:
- Quantify your DNA using the Nanodrop spectrophotometer.
- If the DNA concentration is greater than 200 ng/ul, dilute to 200 ng/ul. I find it useful to set up these dilutions on a 96-well plate. If the DNA is between 50 and 200 ng/ul, just put an aliquot directly on your dilution plate. If it is less than 50 ng/ul, you need to redo the extraction or find a way to concentrate the DNA.
Quantify your ≤200 ng/ul dilution plate using Picogreen:
- Take the tube of bright orange Picogreen reagent out ahead of time to thaw. Wrap it in aluminum foil to protect it from light. It is in DMSO instead of water, so it takes a long time to thaw and will immediately freeze solid if you put it on ice.
- The Quant-iT Picogreen kit comes with a lambda DNA standard at 100 ug/ml. Dilute some of the 20X TE that comes with the kit to 1X TE, and use it to make a 2 ug/ml dilution of the lambda DNA. (1:50 dilution.)
- For one plate (88 samples, 8 standards) make up 20 mL of 1X TE. (1 mL of the TE that comes with the kit, plus 19 mL sterilized filtered water.)
- The plate you need for the assay is a black, flat-well plastic plate. (Corning makes these.)
- Set up a standard curve in column 1 (or column 12, doesn’t matter). Pipette 100 ul of TE into wells B-H. Add 100 ul of your 2 ug/ml lambda standard each to well A and B. Pipette well B up and down to mix, then transfer 100 ul to well C. Pipette well C up and down to mix, then transfer 100 ul to well D. Continue through well G, and leave well H as a blank. (After mixing well G, you will simply throw out 100 ul.)
- Add 99 ul TE to the other 88 (or however many samples you are doing) wells . Add 1 ul of ≤200 ng/ul sample DNA to each well.
- Add 50 ul of Quant-iT reagent to 10 mL of 1X TE. This solution needs to be used within a few hours, even if it is protected from light. Add 100 ul of the solution to each well (both sample, standard, and blank).
- Picogreen bonded to dsDNA has an excitation maximum at 480 nm and emission maximum at 520 nm. The plate readers in IGB (BioTek Synergy HT) probably already have a picogreen program on them.
- Read fluorescence intensity on the plate reader, and export it to Microsoft Excel.
- Make a scatterplot of fluorescence intensity of the standard vs. the standard concentration. Given that the samples were diluted 200X, the standard concentration is multiplied by 200:
- Well A 200 ng/ul
- Well B 100
- Well C 50
- Well D 25
- Well E 12.5
- Well F 6.25
- Well G 3.125
- Well H 0
- In Excel, fit a trendline to the scatterplot and display the equation on the chart. Use this equation to estimate the concentration of the samples.
In most cases, the concentration estimate via Picogreen should be lower than the concentration estimate via Nanodrop. This is because Nanodrop measures DNA + RNA, whereas Picogreen only measures DNA. Why didn’t we just use Picogreen to begin with? Because it can measure a much narrower range of concentrations than Nanodrop can. If the standard curve were any more concentrated, it would not be linear.
Based on the Picogreen concentration estimates, dilute the DNA to 50 ng/μL in 10 mM Tris (and 0.1 mM EDTA, optional).
Notes for samples of concentration lower than 50 ng/μL:
- If you have a lot of samples that are 30-50 ng/μL, you can dilute all samples for your library to 30 ng/μL or 40 ng/μL instead of 50. The amount of adapter that you add at the ligation step (see below) should be reduced proportionately.
- For samples in the 10-50 ng/μL range, a cheap and efficient way to concentrate them is by isopropanol precipitation:
- Combine 200 μL DNA sample, 20 μL 3M sodium acetate, and 200 μL isopropanol.
- Mix well by inversion. Place in the freezer for at least an hour.
- Spin down 10 minutes in the centrifuge.
- Pour off the liquid, taking care to keep the pellet.
- Add 200 μL 70% ethanol to rinse. Invert a few times.
- Spin down 1 minute, then pour off the ethanol, again being careful not to lose the pellet.
- Allow to dry on the lab bench.
- Resuspend the DNA in 20 μL TE.
- Requantify with Picogreen, then dilute to 50 ng/μL.
Restriction digestion and ligation
Restriction digestion master mix:
| Ingredient | For one sample | For one plate |
|---|---|---|
| 50 ng/ul DNA | 5 ul | - |
| 10X NEBuffer 4 | 1.5 ul | 165 ul |
| PstI-HF, 20,000 U/mL | 0.25 ul | 27.5 ul |
| MspI, 20,000 U/mL | 0.25 ul | 27.5 ul |
| Nuclease-free water | 8 ul | 880 ul |
(I have also used DNA at a concentration of 100 ng/ul because that was what Keck wanted for GoldenGate, so then I used 2.5 ul DNA and 10.5 ul water.)
Do this in a 96-well plate. Pipette the DNA into the wells and then add 10 ul of master mix to everything. Pick one well that will not have DNA in it. This will be an important control later on to demonstrate that this library was not contaminated with another library (which will have a different empty well).
Run the Digest program on the PCR machine: 3 hours at 37°C, then 20 minutes at 80°C.
Using a multichannel pipette, add 1.5 μL of 0.1 μM PstI adapters to their corresponding wells on the digestion plate. (Do add the adapter corresponding to the well that has no DNA in it.)
Ligation master mix, keep on ice until use:
| Ingredient | For one sample | For one plate |
|---|---|---|
| 10X Ligase buffer with ATP | 1 ul | 110 ul |
| 10 μM MspI adapter | 0.5 ul | 55 ul |
| 10 mM ATP | 1.5 ul | 165 ul |
| T4 Ligase, 2M U/mL | 0.1 ul | 11 ul |
| Nuclease-free water | 5.4 ul | 594 ul |
Add 8.5 μL of ligation master mix to each well of the digestion plate.
Run on the "ligate" program on the PCR machine: 2 hours at 25°C, 20 minutes at 65°C.
Cleanup and amplification
- Using a multichannel pipette and a PCR 8-well strip tube, pool all the columns together, adding 5 μL from each well of the plate to the wells on the strip tube.
- Pipette the 60 μL out of each well on the strip tube into one 1.5 mL tube. Mix well so that all samples are combined evenly. Freeze or keep on ice.
- Pour a 2% agarose gel with ethidium bromide. Make it nice and deep; my recipe is 3 g agarose, 150 mL 1X TAE, and 7.5 μL ethidium bromide solution. Use a wide-toothed comb.
- Take 40 μL (or more depending on your well volume) of your pooled library and combine it with a loading dye that does not have bromophenol blue. I actually use 10 μL of Promega GoTaq Green PCR buffer, despite the fact that I'm not doing PCR, since it doubles as a loading dye and lacks bromophenol blue.
- I recommend cleaning out your gel rig and putting in fresh TAE, since you especially want to avoid any contamination from other Illumina libraries.
- Run your ~50 μL of library plus loading dye on the gel. The lane with the library should have a lane of 100 bp ladder on either side of it. You can put multiple libraries on one gel, but leave several empty lanes between them.
- The gel doesn't need to be run very long. I would go 20 minutes at 100 V, or until the ladder bands below 500 bp are distinguishable.
- The library should look like a smear. There may be some undigested DNA (a band in the 10's of kb) but that is okay as long as most of the DNA is digested. There may also be a thick band of leftover adapter below 100 bp.
- Using a clean razor blade for each library, cut out the smear between 200 bp and 500 bp. There should definitely be DNA visible in this range.
- Use the Qiagen gel extraction kit to purify the DNA out of this gel slice. Elute in the lower volume (30 μL EB).
- Run the Illumina PCR:
- 3 μL gel-extracted library
- 2 μL 10 μM forward + reverse Illumina primers (PCR1 and PCR2)
- 25 μL 2X Phusion Master mix
- 20 μL nuclease-free water
- PCR program:
- 98°C 30 seconds
- 15 cycles of 98°C 10 seconds, 65°C 30 seconds, 72°C 30 seconds
- 72°C 5 minutes
- Run 5 μL of the PCR product out on a 2% agarose gel. Look to see whether there is primer-dimer visible.
- If there is primer-dimer visible, run the remaining 45 μL of PCR product on a 2% agarose gel and extract the library (as was done pre-PCR). Follow the instructions in the Qiagen gel extraction kit as specified for sequencing. (After binding DNA to the column, do a wash with QG. When rinsing with PE, let sit for 2-5 minutes before spinning.)
- If there is no primer-dimer visible, use the Qiagen PCR cleanup kit to purify the remaining 45 μL of PCR product.
Quality control
- Quantify the purified PCR product using the Picogreen protocol as above. Expected concentrations are in the 10's of ng/μL.
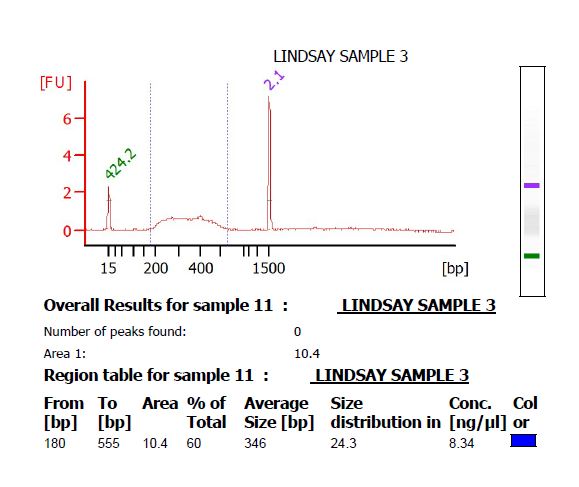
- Run on a DNA 1000 chip on the Bioanalyzer. There should be a smooth curve from around 200 to 500 bp. Any sharp peaks could indicate that the enzymes were cutting in a repetitive region of the genome, in which case it is best to choose different enzymes. Use the Bioanalyzer software to calculate the average fragment size.
- If there is primer-dimer remaining in the library, it will be visible as a sharp peak at a lower molecular weight than the broad peak for the library. (The library pictured below does not have primer-dimer.)
- Calculate the concentration of the PCR product in nM. Keck supplies a worksheet for this calculation. If [math]\displaystyle{ x }[/math] is the concentration in ng/μL, [math]\displaystyle{ y }[/math] is the average size in base pairs, and [math]\displaystyle{ z }[/math] is the concentration in nM, then [math]\displaystyle{ z = \frac{10^6*x}{649y} }[/math].
- Dilute the purified PCR product to 10 nM in EB (10 mM Tris).
- Give 20 μL of 10 nM library to the core facility (Keck). They will use real-time PCR to confirm a concentration of 10 nM. Using Illumina Hi-Seq, do one lane of 100 bp single-end reads.
Bioinformatics
We are aligning Miscanthus sequences to the Sorghum genome, which can be downloaded at phytosome.net.
We are primarily using the software package Stacks for processing the data.
Computation is done on Biocluster, a Unix cluster at the University of Illinois.
Example script for initial processing of the data: Media:LVC121113preprocess.txt
Notes
Please feel free to post comments, questions, or improvements to this protocol. Happy to have your input!
- List troubleshooting tips here.
- You can also link to FAQs/tips provided by other sources such as the manufacturer or other websites.
- Anecdotal observations that might be of use to others can also be posted here.
Please sign your name to your note by adding '''*~~~~''': to the beginning of your tip.
References and additional reading
- Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, et al. (2008) Rapid SNP Discovery and Genetic Mapping Using Sequenced RAD Markers. PLoS ONE 3(10): e3376. doi:10.1371/journal.pone.0003376
- Catchen JM, Amores A, Hohenlohe P, Cresko W, and Postlethwait JH (2011) Stacks: building and genotyping loci de novo from short-read sequences. G3: Genes, Genomes, Genetics 1:171-182. doi: 10.1534/g3.111.000240
- Davey JL and Blaxter MW (2010) RADSeq: next-generation population genetics. Briefings in Functional Genomics 9(5):416-423. doi:10.1093/bfgp/elq031
- Davey, J. W., Cezard, T., Fuentes-Utrilla, P., Eland, C., Gharbi, K. and Blaxter, M. L. (2012), Special features of RAD Sequencing data: implications for genotyping. Molecular Ecology. doi: 10.1111/mec.12084
- Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, and Mitchell SE (2011) A robust, simple Genotyping-by-Sequencing (GBS) approach for high diversity species. PLoS One 6(5): e19379. doi:10.1371/journal.pone.0019379
- Hohenlohe PA, Catchen J, Cresko WA (2012) Population Genomic Analysis of Model and Nonmodel Organisms Using Sequenced RAD Tags. In: Data Production and Analysis in Population Genomics, Pompanon F and Bonin A, eds. 235-260. doi:10.1007/978-1-61779-870-2_14
- Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double Digest RADseq: An Inexpensive Method for De Novo SNP Discovery and Genotyping in Model and Non-Model Species. PLoS ONE 7(5): e37135. doi:10.1371/journal.pone.0037135
- Serang O, Mollinari M, Garcia AAF (2012) Efficient Exact Maximum a Posteriori Computation for Bayesian SNP Genotyping in Polyploids. PLoS ONE 7(2): e30906. doi:10.1371/journal.pone.0030906
The basics
- An overview of restriction digestion and ligation: [1]
- DNA ligation
- Restriction digest
Contact
or instead, discuss this protocol.