Sbb14 Kevin Fan: Difference between revisions
| Line 4: | Line 4: | ||
[[Image:4_24_14_Gel_pdtvectdig.jpg]] | |||
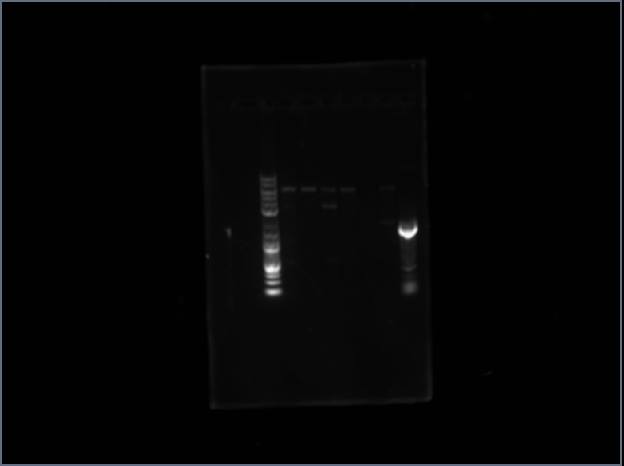
Analytical Gel 1 order: Vectdig Trp1 Trp2 Tsquare TΔ Tx Arg1 Arg2 Arg3 Ladder | |||
Looks like my Thr colonies are all bad. | |||
==[[User:Jiang Lan Fan|Jiang Lan Fan]] 11:56, 22 April 2014 (PDT)== | ==[[User:Jiang Lan Fan|Jiang Lan Fan]] 11:56, 22 April 2014 (PDT)== | ||
Revision as of 11:47, 24 April 2014
~~!~~
Jiang Lan Fan 10:33, 24 April 2014 (PDT)
Analytical Gel 1 order: Vectdig Trp1 Trp2 Tsquare TΔ Tx Arg1 Arg2 Arg3 Ladder
Looks like my Thr colonies are all bad.
Jiang Lan Fan 11:56, 22 April 2014 (PDT)
Digested and Zymoed Tcircle, Tsquare, TX, and TΔ
Gave my Vsquare to Chris for sequencing.
Jiang Lan Fan 11:37, 17 April 2014 (PDT)
Ran analytical gel on our digested vector products!
EMPTY EMPTY LADDER Tsquare Tcircle Vsquare Vcircle Ysquare Ycircle Akash's_PCR'd_Q
Vsquare has 3 bands! Matches with what we want: There's an NcoI internal restriction site that cuts the part into 400 and 2000 bases. Ycircle looks good too. Nothing for T.
Jiang Lan Fan 10:56, 15 April 2014 (PDT)
Digested 2 of each of our miniprepped vector products.
Zymo Cleanup of our digested product vector samples, because we couldn't get a gel box.
Jiang Lan Fan 11:34, 10 April 2014 (PDT)
Today we did miniprep on our 4 colonies per a.a. to purify the plasmids. Put into fridge.
Jiang Lan Fan 10:43, 8 April 2014 (PDT)
Today I isolated 4 colonies into test tubes of 5mL LB/Amp for both of my transfected E. coli plates. Akash did the same for his TyrS-infected E. coli.
Jiang Lan Fan 12:28, 4 April 2014 (PDT)
Successful plates!!!!!
From left: (1) ThrS Transform plate: ~170 colonies (2) ValS Transform plate: ~300 colonies (3) Akash's TyrS Transform plate: ~100 colonies
Our control plate from before (with just the vector digest transformed into cells) had 0 colonies, so that control passed.
Wrapped these plates in Parafilm and put into refrigerator.
Jiang Lan Fan 11:04, 3 April 2014 (PDT)
My plates were crap, so we're redoing ligation and transformation again.
Ligated vectdig with Thrdig Ligated vectdig with Valdig Add cells, heat shock, plate.
Jiang Lan Fan 10:54, 20 March 2014 (PDT)
Finishing up our Zymo Gel Purification on our vectordig.
Ligation of vectdig with my Thrdig and Valdig
Transformation by heat shock: THE FOLLOWING PROCEDURE IS GOOD FOR 2 TRANSFORMATIONS
1. Thaw a 100 uL aliquot of cells on ice 2. IGNORE WATER ADDING STEP 3. Add 35 uL of KCM to the cells 4. Put your ligation mixture on ice, let cool a minute or two (for Miniprep product, dilute by 10, then use 1uL of dilution) 5. Add 65 uL of the cell cocktail to the ligation, stir to mix 6. Let sit on ice for 10 min 7. Heat shock for 90 seconds at 42 (longer incubation may work better) 8. Put back on ice for 1 min 9. Add 100uL of 2YT, let shake in the 37 degree incubator for 1 hour 10. Plate 70+ uL on selective antibiotics, let incubate at 37 degrees overnight
Also made a negative control (ligation without insert).
We plated ThrS, ValS, SerS, ArgS, TrpS, and our negative control onto Ampicillin agar plates.
Jiang Lan Fan 11:53, 18 March 2014 (PDT)
Digest our miniprepped vector:
30uL digest: (15uL vector, 3uL NEBuffer2, 1.5uL NcoI, 1.5uL EcoRI, 9uL ddH2O) at 37°C for 1hour
Run gel purification, 3 wells of 10uL vectdig + 3uL dye.
All three wells worked! Cut out the band with the longer DNA and placed in tube with 600uL ADB buffer. Melted, then frozen.
Jiang Lan Fan 12:10, 13 March 2014 (PDT)
Digestion of Val with BsmBI (NEBuffer 3, 55°C)
Regular Zymo cleanup on first Thr PCR product (the one that ran on 2K55, (1 Thr 2)), labeled (G2 zymo Thr)
Digestion of Thr with NcoI, EcoRI (NEBuffer 2, 37°C)
Regular Zymo cleanup of Valdig, labeled (G2 Val zymodig)
Regular Zymo cleanup of Thrdig, labeled (G2 Thr zymodig)
Jiang Lan Fan 11:20, 11 March 2014 (PDT)
Run gel for our re-dos. I ran my two Thr PCR products.
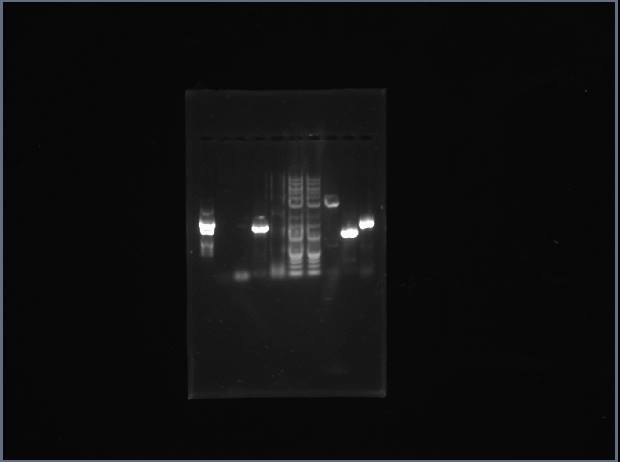
Our second gel:
From left to right:
Christy's Met (2kb), Adriana's 2 pieces of Met for sowing (1.2kb, 0.6kb), Neeka's 2 Pro (ladder in between), Daniel's 2 Arg, Kevin's 2 Thr
Looks like both of my Thr PCRs worked!
Jiang Lan Fan 13:51, 7 March 2014 (PST)
Gotta redo the ThrS PCR.
Made 2 tubes: (1 Thr 2) and (2 Thr 2)
(1 Thr 2) will use 2K55
(2 Thr 2) will use 4K55 again.
Zymo Cleanup: cleaned up my successful ValS PCR product tube using zymo cleanup procedure
Jiang Lan Fan 11:45, 6 March 2014 (PST)
OUR PCRs FINISHED!
Prep of Gel today.
Tomorrow we will do Zymo Cleanup.
Our Gel:
(From left to right: P Q R S T Ladder Ladder V W Y)
Looks like P, Q, R, and T are no bueno.
Jiang Lan Fan 11:52, 4 March 2014 (PST)
Set up PCR for ValS and ThrS (T. thermophilus).
Used protocol "The Basic PCR for Cloning" in PCR+in+Practice.doc
ValS and ThrS both use 4K55 program.
PCR will run overnight or something.
Jiang Lan Fan 10:11, 27 February 2014 (PST)
1) Pouring a gel:
melting agarose in water:
calculate amount of agarose to make a 1% agarose gel
50x buffer in water.
put in microwave, very loosely capped, til you see it start to melt, then cap, shake, then loosen cap to microwave it to boiling.
put the cancerous bromide thing into gel. It comes in a 10000x vial.
Don't shake the gel anymore! stir gently.
Putting gel in apparatus:
put the wells (plastic) into the apparatus, then pour gel into apparatus and wait for it to solidify.
2) Miniprep:
Minipreps can purify DNAs that are under 100kb. Bigger DNA gets lost.
In the box: P2, N3, PB, PE, P1 buffers. Opening up a new kit, need to prepare P1 buffer by adding RNAse then put in fridge. PE buffer needs ethanol added.
Put 1.5mL of cell juice into the small blue tube.
Centrifuge to make cell pellet.
Shake out the supernatant! Make sure pellet is dry or else miniprep will fail!!!
Add 250microL P1 buffer. (Contains a little bit of Tris and RNAse)
P2 has a lot of detergent at high pH (will blow apart Ecoli, as well as proteins+DNA noncovalent bonds)
Add 250 microL P2 (brings pH up to 12) then immediately shake lightly. Solution turns clear real quick.
Neutralize within 20 minutes with 300microL N3 --> drops pH down to 4 or 5.
Shake til miso soup consistency (not egg drop), and centrifuge for 5 min.
Why are we not worried about RNA bleeding through? We added RNAse with the P1 buffer. Also, RNA degrades itself at pH 12.
Pour into new membrane column and centrifuge for 15s.
500microL PB buffer will purify by redissolving everything but plasmid. Another quick spin.
Lastly, Adding 750microL PE buffer (which is basically ethanol), washes out any salts.
Centrifuge for 15s, then what's left on the membrane should be just vector, water, and some ethanol. Spin it again to get rid of moisture.
GET A NEW COLLECTING TUBE
Put 50microL ddH2O right on the middle of the membrane to redissolve the DNA.
Take the liquid and throw away membrane. Done!
3) Running miniprep in gel
Our electrodes are red = positive, black = negative. DNA is negative so it runs towards positive. Remember: Run to Red.
Our voltage depends on how far away our electrodes are (how big our apparatus is).
Run at 160volts.
Pull out the plastic thing from the gel container slowly.
Add 5microL of the marker into a well
Load your samples (with mixed 2microL dye and 5microL water)
RUN THE GEL
Jiang Lan Fan 11:22, 20 February 2014 (PST)
Researching on my two amino acid tRNA synthetases to see if there are any issues with them. Apparently, Valyl-tRNA synthetase binds to Threonine with a certain rate.
Some papers on this: (1 2 3 4 5)
Should be OK though, because the enzymes have domains that can proofread!
Speaking of proofreading, our group posted all of our oligos on a google doc, and we proofread each other's oligos.
Jiang Lan Fan 10:18, 18 February 2014 (PST)
Because there are no internal restriction sites in thrS, my oligos are the following:
> thrS-F using Nco1 restriction site (that cuts ---C CATGG---)
ccaaaCCATGGcggtctacctgccggac
> thrS-R using EcoR1 restriction site (that cuts ---G AATTC---)
gctagGAATTCctaaaagaccggctcaagccg
However, since there is an Nco1 restriction site in valS, I used BsmB1 instead of Nco1:
> valS-F using BsmB1 restriction site (that cuts ---CGTCTCN NNNN---)
ccaaaCGTCTCaCATGgacctgcccaaggcctac
> valS-R using BsmB1 restriction site (that cuts ---CGTCTCN NNNN---)
gctagCGTCTCgAATTctcaccctatttggctgagggc
The BsmB1 site works because the 5' sticky end of the BsmB1 oligo digest will be CATG, same as Nco1 sticky end.
Looking for our vector:
GSI found it for us! pBAD/Myc-His (page 12,13,20)
Jiang Lan Fan 13:08, 14 February 2014 (PST)
I'm in charge of creating construction files (and making oligos) for Threonine and Valine of Thermus thermophilus HB8.
Found them!
Checked both in the complete HB8 genome and both sequences checked out. (Indeed, they are both in the complete genome. Note: valS was in reverse complement in the genome.)
Checking Nco1 site at the beginning of genome:
thrS: start: atgacg = Met Thr. If we change that to atggcg, it would become Met Ala. It doesn't really matter so I'll change atgacg to atggcg.
valS: start: atggac No need to change because it works!
Checking Restriction Sites within genes:
No Nco1 or EcoR1 sites in thrS woooo!
1 Nco1 site in valS, no EcoR1 sites.
Jiang Lan Fan 11:10, 13 February 2014 (PST)
--NcoI---YFG---EcoR1--
CCATGG start codon within restriction enzyme site of NcoI
10% of the time, GTG start codon; 1% of the time TTG start codon (so don't self-annotate the sequence. Use existing annotations) In these 11% of cases, we should just change it to ATG. For the case of GTG, we can just add a Met to the beginning (CCATGG)TG...
Our Team's Task: Make oligos for: [M N P Q R S T V W Y] in genome
All we need is to find the ORF. Easiest is to find PROTEIN then backtrack to DNA. Keep in mind that it could be reverse complement.
What if NcoI site is in the gene one time? We can use sewing (Tutorial 2 style)
What if NcoI site is in the gene multiple times? We can use Bsa1 or Bsmb1 (Golden Gate Rxn style)
Protocols are based on oligos in a 10 micromolar dilution. If we start with 23 nmol of frozen oligo, we must first add water til 100 micromolar, then make a 10-fold dilution.
PCR: use expand polymerase. Refer to Media:PCR+in+Practice.doc for protocol. Template should just be the genomic DNA from ATCC.org
After PCR, do a gel to verify a successful PCR.
Purify: Gel Purify then Zymo Cleanup. Protocol on wiki.
Digest: PCR prod + NEB Buffer + Enzymes + Water
Vector Digest
Ligation: Insertdig + Vectordig + water + Ligase buffer + Ligase (add last)
Transformation: Mix with cells, heat shock, rescue (not really necessary because our vector has AmpR), plate.
Select several colonies, then do colony PCR to screen them.
Profit.