Shlo/notebook
Week 3
Week 2
7/01
Did another miniprep of some Biobricks that Perry had grown in liquid culture. The tubes are in the freezer at my workstation. Perry helped me visualize the Egel from yesterday (the machine is somewhat broken ... I had left the gel in the fridge overnight. Though some of the gel diffused, the bands are still distinguishable). Will post analysis soon.
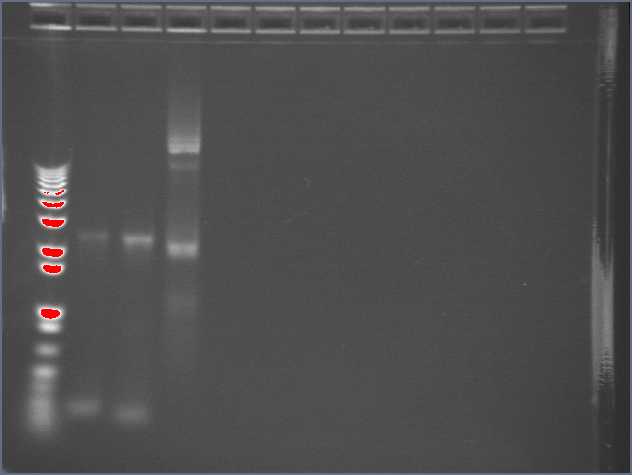
6/30
Helped miniprep some Biobricks, but 3 of the labels smeared and I was unable to discern them. PCR'd them, Will run an E-gel.
E-gel:
Lane 1: 1kb+ ladder
Lane 2: Sample 1
Lane 3: Sample 2
Lane 4: Sample 3
Rest of lanes loaded with water as instructions dictate.
6/29
... oh lord. i have so much more to add. sorry for the delay guys ... mad busy day.
George, Perry, and I arrived at 8:15am to start to prepare the bacteria for the FACS appointment at 3:30. We took the optical densities of the cells we grew in liquid medium overnight and got 1.785, 1.793, 1.780.
9am: We prepared 3 tubes to 2mL each, with dilutions of 1:40, 1:80, 1:100. 2uL of Amp was added in each, and the following:
1:40 - 50uL cells, 1498uL LB
1:80 - 25uL cells, 1748uL LB
1:100- 20uL cells, 1792uL LB
9:15am: Made and set a 1% agarose gel for the digest we prepared yesterday.
Also helped Perry with some of his colony-PCRs. Gel has 3 lanes- 1: ladder, 2: yesterday's digest, 3: Perry's sample. Will be visualized using SyberGold
10:30am: OD of 4-hr samples. Realized we had not diluted them enough. Diluted 1:100 for the spectrometer (so we did not use up too many samples), had reading of 0.03 for 1:40, 0.018 for 1:80, 0.013 for 1:100. Decided to re-dilute 1:100 for samples originally marked 1:40 and 1:100, and 1:30 for sample originally 1:80. Gives us an absolute OD of 0.03 for the 1:40, 0.01 for the 1:100, and 0.05 for the 1:80. (made 2mL total for each sample, adding in cells, LB, and Amp)
1:40- 20uL cells, 2mL LB, 2uL Amp, 1:80 - 65uL cells, 2mL LB, 2uL Amp, 1:100 - 20uL cells, 2mL LB, 2uL Amp.
Also: prepared 2hr samples
11am: George prepares HSL solution using 200proof ethanol.
11:30am: Prepare the 1-hr incubations
12 noon: OD of 4-hour induction, right before protein added. 1:100 dilution done for spectrometer. (10uL cells, 990LB). The numbers below are the raw numbers from the machine - so our samples actually have OD 100x this.
1:40 - 0.002
1:80 - 0.002
1:100 - 0.001
Since we didn't have as much volume of cells as we had anticipated, instead of doing 2mL cell samples as planned, we used 1mL of cells with 10.5 uL HSL (diluted by George - see his personal notebook page). 2 samples were taken from the 1:40 first dilution (8:30am) - used for the 10 and 100nM HSL runs - and 1 from the 1:80, with 1nM HSL.
1:30pm: Induced the two-hour samples. First, OD taken of 2-hr and 1-hr (1hr had been growing for about 1.5 hours at this point).
Again, all ODs diluted 1:100 first.
Two hours: 1:40 - 0.078, 1:80 - 0.035, 1:100 - 0.005. One hour: 1:40 - 0.016, 1:80 - 0.008, 1:100 - 0.007. Also, we had left the 4-hour cells on the bench (from before) - ODs of 0.002 (1:80) and 0.004 (1:100).
2 samples from the 2-hour 1:100 (0.005 OD) were used to make the 10, 100nM HSL.
Since the ODs were already so high for the 1-hr, we also decided to take our original overnight liquid cultures and dilute them for the 1-hr. We used the same formula as above and diluted to 1:80 and 1:100.
2:30pm: 1-hr rediluted samples gave 0.003OD (1:80) and 0.002 (1:100). We used the 1:80 for the 1nM HSL and the 1:100 for the 10, 100nM HSL.
A negative control was also created from 1mL of the 1:80 solution.
3:15pm: All samples spun down for 2 minutes at 10,000rpm, supernatant removed, reconstituted with 0.5mL PBS (1x)
Colony counts from 06/28:
275uL
OmpA1 + 10mer: 167
OmpA1 + 15mer: 266
OmpA1 + 20mer: 224
OmpA2 + 10mer: 60
OmpA2 + 15mer: 9
OmpA2 + 20mer: 28
10uL
OmpA1 + 10mer: 15
OmpA1 + 15mer: 8
OmpA1 + 20mer: 0
OmpA2 + 10mer: 1
OmpA2 + 15mer: 0
OmpA2 + 20mer: 0
6/28
George, Perry, and I transformed some of the BioBricks into 20uL of Top Ten cells.
We want to change the pL promoter because it does not allow for constitutive expression. Some of the Biobrick parts we're using may facilitate this.
Also: grew cells in liquid media, digested BB, looked at sequences and discovered that the BB isn't what it should be - should be about 800bp, with ribosome binding region and terminator region, but was only about 300bp, with terminator and something following it.
6/27
Yesterday night I left some vectors & plasmids to ligate in the 37C incubator. Kevin took the vectors for nucleotide removal, and I speed Vac'd the plasmids. Originally I was going to make a gel and run the plasmids on multiple lanes for gel extraction, but given the large Nanodrop values (242 - 371 ng/uL) and the large amount of Midiprep used (50uL), Bill suggested that I PCR purify them instead, which gives 95% recovery rather than 80% recovery. A gel will still need to be run to confirm that the samples were indeed ligated.
The plasmids are Lpp+OmpA1+pet29 and Lpp+OmpA2+pet29.
- Reconstitution into 30uL of H2O per plasmid (two plasmids total - OmpA1, OmpA2),
- split into two samples each (so 15uL total volume)
- PCR purified (PCR purification kit from Qiagen)
- Eluted with 50uL nuclease-free H2O per tube
- prepared a gel using TBE
- prepared 5 lanes
- Lane 1: 10uL ladder (1kB +)
- Lane 2: 1uL OmpA1 plasmid (diluted to 20uL total using H2O)
- Lane 3: 3uL OmpA1 plasmid (diluted to 20)
- Lane 4: 1uL OmpA2 plasmid (diluted to 20)
- Lane 5: 3uL OmpA2 plasmid (diluted to 20)
The gel was run at 130V for 45 minutes.
4 tubes total should remain: 2 tubes of each type of plasmid. 2 tubes should have 49uL left (labeled "1"), 2 tubes should have 47uL left (labeled "2"). After gel ran for 45 minutes, bands appeared about 3/4 way down the gel. Ethidium bromide (2uL) and 100mL TBE buffer used, put on shaker for 45 minutes. Visualized under Trans-UV.

Nanodrop, 6/27:
10mer: 122.3 ng/uL
15mer: 156.3 ng/uL
20mer: 167.4 ng/uL
plasmidOmpA1: 39.6 ng/uL
plasmidOmpA2: 42.8 ng/uL
We Speed Vac'd the plasmids and resuspended both in 33uL of nuclease-free H2O. To calculate volumes of sample needed , we approximated that the vector was 5800 in size and the insert was 50. Therefore, we'd need 1200ng of vector and 103ng of insert.
Protocol for Ligation of Plasmid given Nanodrop results and volumes available
OmpA1 or A2: 10uL
10mer, 15mer, or 20mer: 1uL
buffer: 1.5uL
ligase: 1uL
dH2O: 1.5uL
(total of 15uL)
Leave in PCR machine (left in the one at George's workstation) overnight for 15C. The extra 10,15,and20mer tubes were put in the "Mike Strong iGEM plasmid" box in the freezer in the small laboratory. We used shorthand for the small PCR tubes: 110 means OmpA1+10mer, 115 means OmpA1+15mer, 210 means OmpA2+10mer, etc.
Readings and Brainstorming
Idea: increasing ligation efficiency
According to a paper I just read, (Lund et al) "temperature cycle ligations" may provide a 4-8 fold increase on cloning efficiency, since the cycling balances high enzyme activity and DNA annealing. The temperature cycle should run for 12-16 hours, cycling between 30 second bouts at 10C and 30C.
Readings toward QS and lux
Bacterial Transformation Experiment James Slock has a detailed protocol for transforming the lux genes into e.coli. Actually, the transformation protocol is the same as what we've been doing, but there is some specific info on lux stuff. Not really essential, I guess.
MIT Parts Registry: Overview of LuxR system
[ww.ai.mit.edu/projects/ cellular-robotics/rweiss-dna6.ps Paper] Really nice, but needs to be converted to pdf - my Mac did this automatically ...
MIT Parts, BBa_F2620
spatiotemporal control reading Look at the supplementary information as well.
Paper ...some other genes that may be turned on by HSL are mentioned ...
somewhat relevant ... but not completely Seems that the e. coli were grown with OHL... and a different strain was used
BL21 More info on the cells we're using
look in methods for treatment of HSL powder ... mammalian cells, but for method only
shlo/notebook/luxfluorescenceduration
Week 1
Doing some reading on Fec now. Really brief notes and some of the more interesting readings follow.
Fec genes encode proteins essential for ferric citrate transport in e.coliK12.
FecA is an outer membrane protein
=N-proximal 79-residue extension: deletion of this extension abolishes induction by ferric citrate but retains feric citrate transport: Kim et al, Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system, and the electrochemical potential of the cytoplasmic membrane Kim article
Gene regulation by transmembrane signaling Some really nice info on structure of Fec and interactions with ferric citrate
Biocyc
- Nakajima H, Shimbara N, Shimonishi Y, Mimori T, Niwa S, and Saya H. Expression of random peptide fused to invasin on bacterial cell surface for selection of cell-targeting peptides. Gene. 2000 Dec 30;260(1-2):121-31. DOI:10.1016/s0378-1119(00)00461-3 |
- Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, and Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002 Jun 13;417(6890):712-9. DOI:10.1038/nature752 |
NanoDrop results (6/20) performed by Ellenor and Stephanie:
(1.5 uL used out of a 30uL elution with nuclease free water)
S: 10.9 ng/uL
S2: 15.4 ng/uL
B: 59.1 ng/uL
B1: 25.1 ng/uL
Brainstorming
Brainstorming for the two-component systems (really for my own use for now - not expected to be coherent)
Structural comparison of the PhoB and OmpR DNA binding/transactivation domains and the arrangement of PhoB molecules on the phosphate box
-NMR used to determine 3DE structure of PhoB DNA-binding/transactivation domain. Very similar to OmpR DNA-binding/transactivation domain, except for conformation of the long turn region of PhoB (interaction site for sigma subunit, rather than interaction with alpha subunit for OmpR)
Interdomain linkers of homologous response regulators determine their mechanism of action
Focuses on OmpR and PhoB and, as the title suggests, supports that phosphorylation of sites (particularly N-terminus of both proteins) improves affinity to bind DNA. Isolated C terminus of OmpR is insufficient to productively interact with RNA polymerase.
I've been told by some of the lab members that OmpR is an inner-membrane protein and therefore cannot be used for our assays. It seems that we'll have to find another protein ...
The phosphoryl transfer domain of UhpB interacts with the response regulator UhpA
UhpB = histidine kinase protein that controls production of sugar phosphate transporter UhpT
UhpA = response regulator; when phosphate is transferred from histidine to aspartate, ability of kinase to bind to target DNA sequences and to alter gene transcription is altered.
Major result: indication that phosphoryl, transfer-dimerization of UhpB participates in specific binding of UhpA, in control of autokinase activity, and dephosphorylation of P-UhpA
... So I found this paper on a search on PubMed for e coli outer membrane protein signaling dimerization. However, I've found that UhpA resides in the cytoplasm, whereas UhpB is an inner-membrane protein. Boo.
More thoughts: we could potentially try to target some of these inner-membrane proteins to the outer-membrane. I don't know if this is really feasible - while we can attach the appropriate signal sequence, I'm not sure the environment would allow for correct conformation and activity of said proteins.