Spring 2006 pCMV-Tag2A / hDlg work: Difference between revisions
No edit summary |
|||
| Line 163: | Line 163: | ||
Performed Topo cloning with 1 ul vector, 1 ul salt solution, 4 ul gel-purified PCR. Transformed Top10 cells and plated onto new amp plates which Alain poured. | Performed Topo cloning with 1 ul vector, 1 ul salt solution, 4 ul gel-purified PCR. Transformed Top10 cells and plated onto new amp plates which Alain poured. | ||
==3/15/06== | |||
Yi-an ran gel of digests for me. | |||
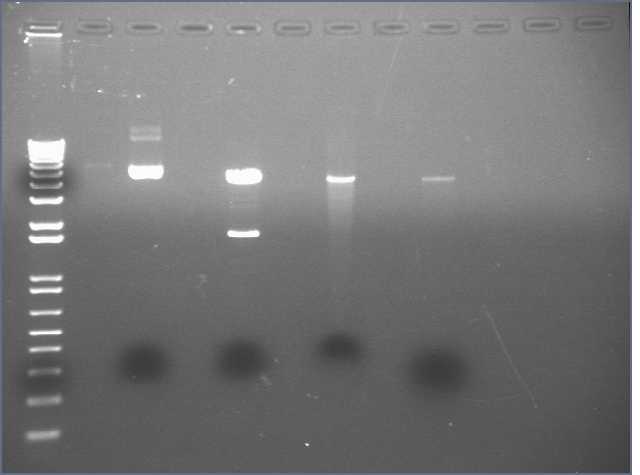
[[Image:3-15 digest PT.jpg]] | |||
Lane 1: 1 kb | |||
Lane 3: Undigested vector | |||
Lane 5: New digest with 3 ul midiprep | |||
Lane 7: Redigest of gel extracted DNA | |||
Lane 9: Gel extracted DNA. | |||
The 2nd band appeared again, and did not match up with supercoiled undigested plasmid. So we suspect that this is an actual insert from the plasmid. pCMV-Tag2 control plasmid (for transfection control) includes an Luciferase insert. | |||
Revision as of 10:20, 15 March 2006
Notes from fall 2005 have been archived into "Perry, fall 2005"
Table 1: Composition of the hDlg constructs
| Construct | Parts |
|---|---|
| LS25G | P1 + P2 |
| LS35G | P1 + P3 |
| LS325G | P1 + P4 + P5 |
| LS254G | P1 + P6 + P7 + P8 |
| LS354G | P1 + P4 + P9 + P8 |
Table 2: Composition and construction of parts
| Parts | Domains | Template | Primers |
|---|---|---|---|
| P1 | L27 | F11 | L27S + L27A |
| P2 | SH3-I2-I5-GK | SG25 | SH3S + GKA |
| P3 | SH3-I3-I5-GK | SG35 | SH3S + GKA |
| P4 | SH3-I3 | SG35 | SH3S + I3A |
| P5 | I2-I5-GK | SG25 | I2S + GKA |
| P6 | SH3 | F11 | SH3S + SH3A |
| P7 | I2-I5-I4 | I2-I5-I4/Topo | I2S + I4A |
| P8 | GK | F11 | GKS + GKA |
| P9 | I5-I4 | I2-I5-I4/Topo | I5S + I4A |
3/3/06
Transformed DH5alpha competent cells (50 ul aliquots) with pcMV-Tag2A vectors. The stock received was 1 ug/ul, used 1 ul for transformation (did two identical ones), 2.5 ul pUC19 for (+) and nothing for (-). On ice for 30 minutes. Heat shock 42 degrees Celsius for 25 seconds. On ice for 2 minutes. Added 950 ul prewarmed SOC, and shook at 225 rpm, 37 degrees, for 1 hour. (followed protocol from DH5alpha product information, except for heat shock step)
Transformed TOP10 competent cells with hDlg-cloned Topo vectors SG25, SG35, and F11. Diluted stock vector 1:10, used 5 ul for transformation, 2.5 ul pUC19 for (+), nothing for (-). On ice for 30 minutes. Heat shock 42 degrees Celsius for 30 seconds, on ice for 2 minutes. Added 250 ul room-temp SOC, and shook at 225 rpm, 37 degrees for 1 hour. (followed previous MCB100 protocol)
Plated bacteria with controls and Topo vectors on carb plates, bacteria with pCMV vectors on kan plates, with glass beads under burner. Placed in 37 degree incubator at 6 pm.
3/4/06
Transformations didn't go great, so Yi-an reperformed them.
3/5/06
hDlg transformations from yesterday didn't show identifiable colonies, more a smear of nothing. pCMV transformations from yesterday produced a few colonies. hDlg transf from Friday were overgrown in SG35 and F11; must have used too much vector to transform.
Prepared three 200 ml LB liquid cultures from single colonies of bacteria transformed with hDlg F11, SG25, and SG35, with 200 ul ampicillin (50 mg/ml); and one 200 ml, pCMV, with 200 ul kanamycin (50 mg/ml). put in shakers around 9:30 pm, set at 300 rpm and 37 degrees Celsius. also streaked onto new plates.
3/10/06
Yi-an already performed the midipreps and PCRs. Today I PCR purified P5-9. Before purification, I took out 10 ul fromeach and added 10 ul water for 1.2% E-gel. I measured the volume left and added 5 volumes Buffer PB to each PCR product. P5 had 20 ul, and I added 100 ul PB; P6, 35, 175; P7, 35, 175; P8, 30, 150; P9, 35, 175. I proceeded with PCR purification, eluting with 30 ul EB. I took out 10 ul from each purified product and added 10 ul water for E-gel. I also took out 10 ul of 1 kb ladder and of 2 log ladder, and added 10 ul water for E-gel.
Lane 1: 1 kb ladder
Lane 2: P5 before
Lane 3: P5 after
Lanes 4-11: P6-9 before and after
Lane 12: 2 log ladder
P5 and P6 have the right lengths.
Setup PCRs for P7-9 again, but this time with undiluted I2-I5-I4/Topo for 7 and 9. Repeated E-gel with 10 ul before and after PCR purification. The volume left for PCR purification was 37 ul in each, and I added 185 ul PB to each.
Lane 1: 1 kb ladder
Lane 2: P7 before
Lane 3: P7 after
Lanes 4-7: P8 and P9 before and after
Lane 8: 2 log ladder
Performed Topo vector ligation using P5, P6, and P8, with 4 ul PCR product, 1 ul Salt solution, 1 ul Topo vector. Incubated room temp 5 min, transferred into tube of chemically competent Top10 cells. Split one 60 ul tube into two 30 ul tubes, one for untreated (-) and one for pUC19-transformed positive (+). On ice for 20 minutes, heat shock at 42 degrees Celsius for 30 seconds, one ice for 2 minutes, added 250 ul SOC media to each tube, shook at 37 degrees Celsius for 1 hour, plated w/ carb.
3/13/06
Transformed one tube of Top10 with 2 ul of original SG35-cloned vector, left one tube untreated for negative. Used 350 ul SOC, and plated a 50, 100, and 200 ul plate from each tube.
Prepared 2 identical double digests of pCMV midiprep: 5 ul pCMV (~213 ng/ml), 2 ul BamHI buffer (10X), 0.2 ul BSA (100X), 0.5 ul BamHI, 0.5 ul XhoI, 11.8 water. Left in PTC-100 at 37 degrees for 6 hours, 80 degrees for 20 minutes, 4 degrees indefinitely.
3/14/06
Ran digest on gel.
Lane 1: 1 kb ladder
Lane 3: pCMV digest #1
Lane 5: pCMV digest #2
The upper band is the correct size for linear vector, and the lower band is probably supercoiled undigested vector.
Performed 2 parallel QIAEX II gel extractions, for DNA >4kb. Preweighed centrifuge tubes at 1.016 and 1.030 g, then weighed with gel slices at 1.108 and 1.161 g. Used 300 and 390 ul QX1, 30 ul QIAEX II each b/c of strong bands, but I forgot to add 2 volumes of water to each. After 50 degree incubation, I added 10 ul 3M sodium acetate to each because of a pinkish color which then turned yellow. Air dried over 30 minutes until about half of each pellet turned from clearish gray to white. Eluted each twice with 20 ul water. Ended up with 2 tubes of ~35 ul elution. Froze one tube, used other tube for a redigestion.
Prepared 3 digests.
1. New digest of pCMV, same as yesterday except with 3 ul DNA and 13.8 ul water.
2. A negative control digest with 3 ul DNA, no enzyme, and 14.8 ul water.
3. A redigest of the DNA that was gel extracted today, using 35 ul gel extraction, 5 ul BamHI buffer, 0.5 ul BSA, 1.25 ul BamHI, 1.25 ul XhoI, and 7 ul water, for a 50 ul digestion.
Ran digest at 37 degrees for 12 hours, 80 degrees for 20 minutes, and 4 degrees indefinitely.
Had to pause the digestion at 3 hours and use PTC machine for annealing BB linkers. 5 ul BBCS1 (for 50 pmol from 10 uM), 5 ul BBCS2, 5 ul T4 ligase buffer (10X), 0.5 ul T4 kinase, 34.5 ul water, at 37 degrees for 30 minutes, 65 degrees for 20 minutes, 94 degrees for 1 minute, let cool to room temperature.
Left digests out at room temp. Returned to PTC after linkers were done and completed 9 more hours of 37 degree incubation.
Helped Yi-an with gel extraction of PCR products. Repeated steps for QUIAEX II protocol, only used 3 volumes of QX1, 10 ul of QIAEX II, added 10 ul sodium acetate two times until yellow. Eluted twice with 20 ul water.
Performed Topo cloning with 1 ul vector, 1 ul salt solution, 4 ul gel-purified PCR. Transformed Top10 cells and plated onto new amp plates which Alain poured.
3/15/06
Yi-an ran gel of digests for me.
Lane 1: 1 kb
Lane 3: Undigested vector
Lane 5: New digest with 3 ul midiprep
Lane 7: Redigest of gel extracted DNA
Lane 9: Gel extracted DNA.
The 2nd band appeared again, and did not match up with supercoiled undigested plasmid. So we suspect that this is an actual insert from the plasmid. pCMV-Tag2 control plasmid (for transfection control) includes an Luciferase insert.