Sriram Lab:Research: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
The Sriram Lab's research is focused on two related areas: metabolic engineering and systems biology. Metabolic engineering is the rational modification of organisms for improvement of their cellular properties. Systems biology is the holistic, quantitative analysis of large-scale biological data sets toward improved understanding, prediction, and control of how a cell or organism behaves. Both these are interdisciplinary fields with immense potential for chemical engineers to uniquely apply their expertise as well as very rapidly growing research areas. | The Sriram Lab's research is focused on two related areas: metabolic engineering and systems biology. Metabolic engineering is the rational modification of organisms for improvement of their cellular properties. Systems biology is the holistic, quantitative analysis of large-scale biological data sets toward improved understanding, prediction, and control of how a cell or organism behaves. Both these are interdisciplinary fields with immense potential for chemical engineers to uniquely apply their expertise as well as very rapidly growing research areas. | ||
[[Image:Labeling expt Sriram.jpg|thumb| | [[Image:Labeling expt Sriram.jpg|thumb|right|520px|'''Quantifying carbon traffic by isotope-assisted metabolic flux analysis.''' Click for detail.]] | ||
We analyze and engineer metabolic and gene regulatory pathways of | We analyze and engineer metabolic and gene regulatory pathways of plant, mammalian and other eukaryotic cells. Metabolic pathways are "traffic maps" of carbon and other elements within cells and gene regulatory pathways are networks showing how this traffic is controlled by the cell. Such analysis provides insights into bottlenecks existing in the cell and how these can be improved by engineering the pathways. We combine experimental techniques such as isotope labeling, two-dimensional (2-D) NMR, gas chromatography-mass spectrometry (GC-MS), DNA microarray analysis and quantitative RT-PCR (qPCR) with several computational techniques for metabolic flux/pathway analysis and deduction of gene regulatory networks. | ||
Studying biological networks in plants quantitatively has much promise for a sustainable future. This is because plants are the primary producers of several commodities crucial to an economy such as food, biofuels, fiber, several high-value therapeutics and recently, chemical industry feedstocks. Highly sophisticated plant metabolic networks synthesize these commodities from thin air (CO<sub>2</sub>), light and minerals. Quantitative studies of plant networks opens the prospect of smartly engineering these networks. Mammalian tissue cultures provide a means to understand human genetic diseases in greater detail, especially how biological networks are perturbed due to the lack of a gene or genes. | |||
{| | |||
| [[Image:Soybean 2D HSQC.jpg|thumb|left|450px|'''Two-dimensional [<sup>13</sup>C, <sup>1</sup>H] NMR spectrum of protein hydrolysate from soybean embryos.''' Click for detail.]] | |||
| [[Image:H4IIE flux map (GK2 2 colors).jpg|thumb|right|270px|'''Global metabolic changes due to glycerol kinase overexpression in rat liver cells.''' Click for detail.]] | |||
|} | |||
</div> | </div> | ||
Revision as of 13:01, 9 August 2009
The Sriram Lab's research is focused on two related areas: metabolic engineering and systems biology. Metabolic engineering is the rational modification of organisms for improvement of their cellular properties. Systems biology is the holistic, quantitative analysis of large-scale biological data sets toward improved understanding, prediction, and control of how a cell or organism behaves. Both these are interdisciplinary fields with immense potential for chemical engineers to uniquely apply their expertise as well as very rapidly growing research areas.
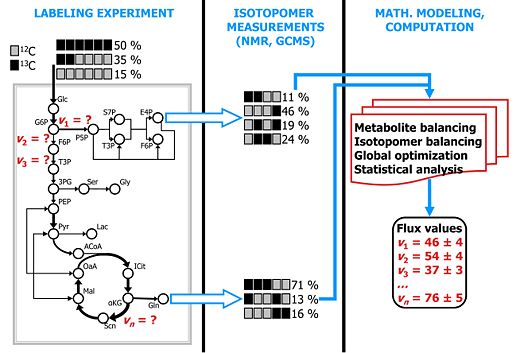
We analyze and engineer metabolic and gene regulatory pathways of plant, mammalian and other eukaryotic cells. Metabolic pathways are "traffic maps" of carbon and other elements within cells and gene regulatory pathways are networks showing how this traffic is controlled by the cell. Such analysis provides insights into bottlenecks existing in the cell and how these can be improved by engineering the pathways. We combine experimental techniques such as isotope labeling, two-dimensional (2-D) NMR, gas chromatography-mass spectrometry (GC-MS), DNA microarray analysis and quantitative RT-PCR (qPCR) with several computational techniques for metabolic flux/pathway analysis and deduction of gene regulatory networks.
Studying biological networks in plants quantitatively has much promise for a sustainable future. This is because plants are the primary producers of several commodities crucial to an economy such as food, biofuels, fiber, several high-value therapeutics and recently, chemical industry feedstocks. Highly sophisticated plant metabolic networks synthesize these commodities from thin air (CO2), light and minerals. Quantitative studies of plant networks opens the prospect of smartly engineering these networks. Mammalian tissue cultures provide a means to understand human genetic diseases in greater detail, especially how biological networks are perturbed due to the lack of a gene or genes.


