Swartz:Research/Biohydrogen: Difference between revisions
No edit summary |
No edit summary |
||
| (25 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Swartz}} | {{Template:Swartz}} | ||
The Biohydrogen team | The Biohydrogen team in the [[Swartz| Swartz Lab]] focuses on developing Biotechnology-based solutions to the global energy problem. This is an important problem: the current method for making hydrogen--steam reformation of natural gas--is energy-intensive and [http://openwetware.org/images/6/68/H2CO2.pdf accounts for 2.3% of the total human emissions of carbon dioxide].Specifically, we are interested in developing sustainable processes for the production of hydrogen from (1) Sunlight though an engineered photosynthetic organism and (2) Biomass using engineered cell free extracts. | ||
The | The Biohydrogen team currently consists of Kunal Mehta, Stacey Shiigi, Franklin Lu, Jamin Koo, Lilian De La Paz, Sylvie Liong, and Marcus Rohovie. Former members of the group include Marcus Boyer, James Stapleton, Jon Kuchenreuter, Phil Smith, and Alyssa Bingham. | ||
{|cellspacing="5" cellpadding="10" style="background:#3674C2; width: 750px;" | |||
|-valign="top" | |||
|style="background:#ffffff"| | |||
<span style="border-bottom: 1px solid #000;">'''1) Biohydrogen from engineered cyanobacteria (Kunal Mehta, Stacey Shiigi)''' | |||
[[Image:cyanobacteria_hydrogen.tif|800px]] | |||
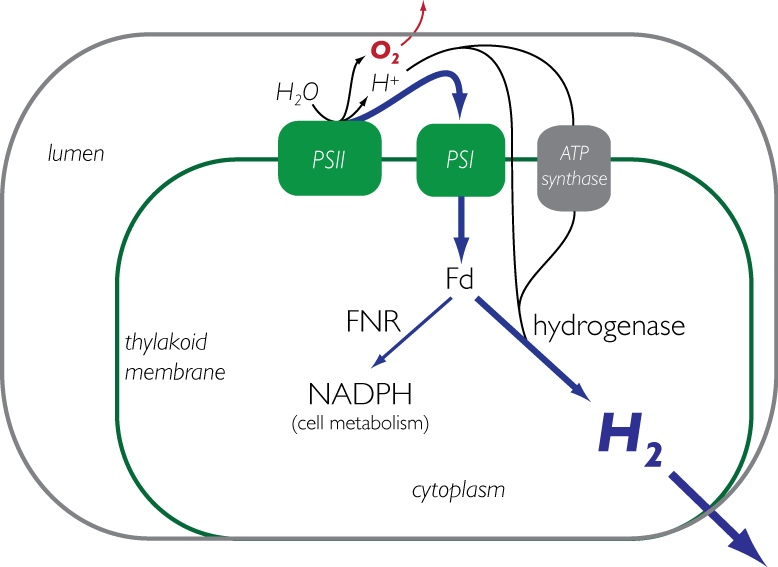
Current methods to produce biochemicals rely primarily on existing sources of stored energy such as glucose that can be converted metabolically into useful end products such as ethanol. However, the production of those sugars are driven from complex chemistry within plants that capture energy from the sun through photosynthesis. The primary goal of this research is to engineer a photosynthetic bacteria to produce hydrogen directly from light. Currently, research is focused on both developing new tools for genetic engineering of cyanobacteria, as well as strain engineering for optimal expression of a synthetic pathway to accept electrons from photosynthetic machinery and redirect them to the production of hydrogen. Along these lines we are also interested in protein engineering of heterologous genes in cyanobacteria to efficiently couple with natural systems. | |||
<span style="border-bottom: 1px solid #000;">'''2) Biohydrogen from cellulosic biomass through engineered cell free extracts (Franklin Lu, Sylvie Liong, Nik Evitt)''' | |||
[[Image:Biomass_to_H2.jpg]] | |||
The | An alternative approach for generation of biohydrogen seeks to capitalize on current research into the efforts to efficiently break down cellulosic biomass including feedstocks such as corn stover which are currently left in fields agricultural residual. The DOE estimates up to a billion tons of material could be available to be converted to useful energy and chemical products. We seek to utilize the sugar units freed from the breakdown of cellulosic biomass which consist primarily of glucose as well as other five carbon sugars such as xylose and convert that to biohydrogen. | ||
In order to maximize the both the yield and minimize the cost, our current research focuses on the generation of cell free extracts that can cheaply and rapidly produce enzymes we need for specific chemical conversions and couple those to a synthetic pathway to generate hydrogen. Consequently we are focused on both development and optimization of a synthetic enzymatic pathway to hydrogen through protein engineering and on cell free metabolic engineering to selectively knockdown and up-regulate required enzymes to maximize yield. | |||
*Smith PR, Bingham AS, Swartz JR. Generation of hydrogen from NADPH using an [FeFe] hydrogenase. ''International Journal of Hydrogen Energy'' (2011) [http://dx.doi.org/10.1016/j.ijhydene.2011.03.172 doi:10.1016/j.ijhydene.2011.03.172] | *Smith PR, Bingham AS, Swartz JR. Generation of hydrogen from NADPH using an [FeFe] hydrogenase. ''International Journal of Hydrogen Energy'' (2011) [http://dx.doi.org/10.1016/j.ijhydene.2011.03.172 doi:10.1016/j.ijhydene.2011.03.172] | ||
*Lu Franklin, Smith Phil, Mehta Kunal, Swartz James. 2015 “Development of a synthetic pathway to convert glucose to hydrogen using cell free extracts.” International Journal Hydrogen Energy 40 (2015) p.9113-9124 [http://www.sciencedirect.com/science/article/pii/S0360319915013257 DOI: 10.1016/j.ijhydene.2015.05.12] | |||
<span style="border-bottom: 1px solid #000;">'''3) Engineering and Maturation of [FeFe] Hydrogenase from ''Clostridium Pasteurianum'' (Jamin Koo, Liliana De La Paz, Marcus Rohovie, Tim Schnabel, Ingmar Burstel)''' | |||
The third major focus of the biohydrogen group is on the shared critical protein for both methods above, an [FeFe] hydrogenase, from the organism ''Clostridium Pasteurium''. Both current and previous students have focused on engineering an oxygen tolerant hydrogenase that has both a high rate of hydrogen formation and resistance to inactivation. These proteins catalyze the interconversion of protons, electrons, and dihydrogen; they are especially oxygen-sensitive due their FeS chemistry.To further these goals we are developing high throughput tools to rapidly screen large libraries for hydrogen production, as well as rationally designing hydrogenase mutations in attempts to confer resistance to oxygen. Simultaneously, we are investigating the complex mechanisms for production and maturation of these enzymes in an effort to better understand the fundamental biochemistry that drives the assembly and activity of these natural hydrogen producers. In this work, we are identifying key components of hydrogenase maturation and in collaboration with the Cramer and Britt labs at UC Davis, are analyzing these changes through EPR and Mossbauer spectroscopy. | |||
*Kuchenreuther JM, George SJ, Grady-Smith CS, Cramer SP, Swartz JR. Cell-free H-cluster Synthesis and [FeFe] Hydrogenase Activation: All Five CO and CN Ligands Derive from Tyrosine. ''PLoS One'' '''6''', e20346 (2011) {{pmid|21673792}} | |||
*Kuchenreuther JM, Grady-Smith CS, Bingham AS, George SJ, Cramer SP, Swartz JR. High-yield expression of heterologous [FeFe] hydrogenases in Escherichia coli. ''PLoS ONE'' '''5''', e15491 (2010). {{pmid|21124800}} | |||
*Kuchenreuther JM, Stapleton JA, Swartz JR. Tyrosine, cysteine, and S-adenosyl methionine stimulate in vitro [FeFe] hydrogenase activation. ''PLoS ONE'' '''4''', e7565 (2009). {{pmid|19855833}} | |||
*Boyer ME, Stapleton JA, Kuchenreuther JM, Wang CW, Swartz JR. Cell-free synthesis and maturation of [FeFe] hydrogenases. ''Biotechnol Bioeng'' '''99''', 59-67 (2008). {{pmid|17546685}} | |||
*Boyer ME, Wang CW, Swartz JR. Simultaneous expression and maturation of the iron-sulfur protein ferredoxin in a cell-free system. ''Biotechnol Bioeng'' '''94''', 128-38 (2006). {{pmid|16570319}} | |||
*Bingham AS, Smith PR, Swartz JR. Evolution of an [FeFe] hydrogenase with decreased oxygen sensitivity. ''International Journal of Hydrogen Energy'' (2011) [http://dx.doi.org/10.1016/j.ijhydene.2011.02.048 doi:10.1016/j.ijhydene.2011.02.048] | |||
*Stapleton JA, Swartz JR. Development of an in vitro compartmentalization screen for high-throughput directed evolution of [FeFe] hydrogenases. ''PLoS ONE'' '''5''', e15275 (2010). {{pmid|21151915}} | |||
*Stapleton JA, Swartz JR. A cell-free microtiter plate screen for improved [FeFe] hydrogenases. ''PLoS ONE'' '''5''', e10554 (2010). {{pmid|20479937}} | |||
Funding: We are grateful for funding from GCEP and the DOE | Funding: We are grateful for funding from [http://gcep.stanford.edu/ GCEP] and the DOE | ||
<html><iframe src="http://www.mendeley.com/groups/1295093/_/widget/0/0/" frameborder="0" allowTransparency="true" style="width:260px;height:200px;"></iframe><p style="width:260px"><a href="http://www.mendeley.com/groups/1295093/swartz-lab-biohydrogen/" | <html><iframe src="http://www.mendeley.com/groups/1295093/_/widget/0/0/" frameborder="0" allowTransparency="true" style="width:260px;height:200px;"></iframe><p style="width:260px"><a href="http://www.mendeley.com/groups/1295093/swartz-lab-biohydrogen/" </a>.</p></html> | ||
Revision as of 16:36, 4 July 2015
| Research | Papers | People | Contact |
The Biohydrogen team in the Swartz Lab focuses on developing Biotechnology-based solutions to the global energy problem. This is an important problem: the current method for making hydrogen--steam reformation of natural gas--is energy-intensive and accounts for 2.3% of the total human emissions of carbon dioxide.Specifically, we are interested in developing sustainable processes for the production of hydrogen from (1) Sunlight though an engineered photosynthetic organism and (2) Biomass using engineered cell free extracts.
The Biohydrogen team currently consists of Kunal Mehta, Stacey Shiigi, Franklin Lu, Jamin Koo, Lilian De La Paz, Sylvie Liong, and Marcus Rohovie. Former members of the group include Marcus Boyer, James Stapleton, Jon Kuchenreuter, Phil Smith, and Alyssa Bingham.
|
1) Biohydrogen from engineered cyanobacteria (Kunal Mehta, Stacey Shiigi) Current methods to produce biochemicals rely primarily on existing sources of stored energy such as glucose that can be converted metabolically into useful end products such as ethanol. However, the production of those sugars are driven from complex chemistry within plants that capture energy from the sun through photosynthesis. The primary goal of this research is to engineer a photosynthetic bacteria to produce hydrogen directly from light. Currently, research is focused on both developing new tools for genetic engineering of cyanobacteria, as well as strain engineering for optimal expression of a synthetic pathway to accept electrons from photosynthetic machinery and redirect them to the production of hydrogen. Along these lines we are also interested in protein engineering of heterologous genes in cyanobacteria to efficiently couple with natural systems.
An alternative approach for generation of biohydrogen seeks to capitalize on current research into the efforts to efficiently break down cellulosic biomass including feedstocks such as corn stover which are currently left in fields agricultural residual. The DOE estimates up to a billion tons of material could be available to be converted to useful energy and chemical products. We seek to utilize the sugar units freed from the breakdown of cellulosic biomass which consist primarily of glucose as well as other five carbon sugars such as xylose and convert that to biohydrogen. In order to maximize the both the yield and minimize the cost, our current research focuses on the generation of cell free extracts that can cheaply and rapidly produce enzymes we need for specific chemical conversions and couple those to a synthetic pathway to generate hydrogen. Consequently we are focused on both development and optimization of a synthetic enzymatic pathway to hydrogen through protein engineering and on cell free metabolic engineering to selectively knockdown and up-regulate required enzymes to maximize yield.
The third major focus of the biohydrogen group is on the shared critical protein for both methods above, an [FeFe] hydrogenase, from the organism Clostridium Pasteurium. Both current and previous students have focused on engineering an oxygen tolerant hydrogenase that has both a high rate of hydrogen formation and resistance to inactivation. These proteins catalyze the interconversion of protons, electrons, and dihydrogen; they are especially oxygen-sensitive due their FeS chemistry.To further these goals we are developing high throughput tools to rapidly screen large libraries for hydrogen production, as well as rationally designing hydrogenase mutations in attempts to confer resistance to oxygen. Simultaneously, we are investigating the complex mechanisms for production and maturation of these enzymes in an effort to better understand the fundamental biochemistry that drives the assembly and activity of these natural hydrogen producers. In this work, we are identifying key components of hydrogenase maturation and in collaboration with the Cramer and Britt labs at UC Davis, are analyzing these changes through EPR and Mossbauer spectroscopy.
Funding: We are grateful for funding from GCEP and the DOE <html><iframe src="http://www.mendeley.com/groups/1295093/_/widget/0/0/" frameborder="0" allowTransparency="true" style="width:260px;height:200px;"></iframe><p style="width:260px"><a href="http://www.mendeley.com/groups/1295093/swartz-lab-biohydrogen/" </a>.</p></html> |