TIP1: Difference between revisions
| Line 61: | Line 61: | ||
=='''Current projects'''== | =='''Current projects'''== | ||
'''Assays of S-acylation''' | '''Assays of S-acylation''' | ||
We have recently developed a method for assaying the S-acylation state of proteins from plants. We have demonstrated that α-tubulin is S-acylated (Fig 8, Hemsley and Grierson | We have recently developed a method for assaying the S-acylation state of proteins from plants. We have demonstrated that α-tubulin is S-acylated (Fig 8, []Hemsley and Grierson) as a proof of concept and are currently assaying the S-acylation state of other proteins in Arabidopsis. [[image:Tubulin_assay.gif|frame|right|Fig 8. Arabidopsis α-tubulin is S-acylated.]] | ||
'''Identification of TIP1 substrates''' | '''Identification of TIP1 substrates''' | ||
No consensus sequence exists for S-acylation making prediction of substrates virtually impossible. We are developing proteomic tools to purify and identify palmitoylated proteins in collaboration with | No consensus sequence exists for S-acylation making prediction of substrates virtually impossible. We are developing proteomic tools to purify and identify palmitoylated proteins in collaboration with Paul Dupree (University of Cambridge, UK) and Kate Heesom (Univesity of Bristol, UK). | ||
'''Effects of S-acylation of protein distribution''' | '''Effects of S-acylation of protein distribution''' | ||
Revision as of 05:47, 23 June 2008
Piers Hemsley
piers.hemsley@bris.ac.uk
S-acylation in plants
Palmitoylation, more correctly known as S-acylation, is a protein modification common to all eukaryotes that allows the reversible association of proteins with membranes.
Arabidopsis thaliana or Thale cress is the best studied and understood model plant system and has a fully sequenced and comprehensively annotated genome. Like animals and fungi, plants utilise S-acylation and the other hydrophobic lipid modifications myristoylation, farnesylation and geranylgeranylation to enable protein association with membranes. Where plants differ is in how they are used.
Prenylation
Geranylgeranylation is used extensively in plants while farnesylation is used much less than would be expected when compared to other systems. Interestingly loss of either or both modifications is not lethal although plants do appear different to wild type (Running et al., 2004,) . This is in marked contrast to fungi and animals where the loss of even just geranylgeranylation is much more serious and frequently lethal.
Myristoylation
Arabidopsis contains two N-myristoyltransferase genes and 0.8% of Arabidopsis proteins are predicted to be myristoylated compared to 0.5% for humans and 0.3% for fungi underlining the relative importance of myristoylation to plant growth.
S-acylation


23 DHHC protein S-acyl transferase genes (AtPATs) are present in the Arabidopsis genome. Currently there are very few known S-acylated proteins in plants although it would appear that, given the number of PATs present, regulation of S-acylation in plants is likely to be an important and complex process. Loss of the TIP1 S-acyl transferase in tip1-2 mutant plants leads to reduction in cell size, plant size (Fig 1.), fertility and growth polarity. The loss of growth polarity is best demonstrated by the shortening and branching of root hairs (Fig 2.) and the erratic and growth of pollen tubes that frequently change their direction of growth. Phenotypes for other AtPAT mutants are emerging from T-DNA screens and affect many aspects of Arabidopsis growth and development (Figs 3 and 4).


S-acyl transferases

S-acyl transferases were first identified in yeast by Nick Davis' lab when they showed that the ankyrin repeat containg protein AKR1 is able to attach palmitate groups to Yeast Casein Kinase 2 (YCK2, (Roth et al., 2002). Subsequently Bob Deschenes' group identified a RAS2 S-acylating enzyme in the form of ERF2 (Lobo et al., 2002). ERF2 lacks the ankyrin repeats of AKR1 but both contain a DHHC domain. All S-acyl transferases characterised to date are polytopic membrane proteins and contain a DHHC motif zinc finger which is thought to be the active site for S-acylation reactions and faces the cytoplasmic side of the membrane (Fig 5.). To date S-acyl transferases have been identified biochemically from most fungal and metazoan model organisms but only TIP1 from Arabidopsis has been characterised from plants.
TIP1
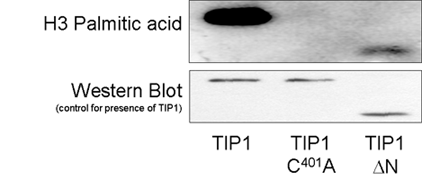
TIP1 is the only one of the 23 Arabidopsis S-acyl transferases to contain an additional recognised domain in addition to the DHHC domain. TIP1 contains 6 ankyrin repeats at the N terminus and is similar to AKR1 from budding yeast and HIP14 from Humans and these proteins form a distinct subset of S-acyl transferases. Domain deletion experiments with TIP1 indicate that the ankyrin repeats are not required for S-acyl transferase activity (Fig 6.). TIP1, and by extension all ankyrin repeat containing S-acyl transferases, may therefore be bifunctional proteins. Introducing these domain mutants into plants indicates that S-acyl transferase activity but not the function provided by the ankyrin repeats is required for polar root hair growth (Fig 7.).

Current projects
Assays of S-acylation
We have recently developed a method for assaying the S-acylation state of proteins from plants. We have demonstrated that α-tubulin is S-acylated (Fig 8, []Hemsley and Grierson) as a proof of concept and are currently assaying the S-acylation state of other proteins in Arabidopsis.

Identification of TIP1 substrates No consensus sequence exists for S-acylation making prediction of substrates virtually impossible. We are developing proteomic tools to purify and identify palmitoylated proteins in collaboration with Paul Dupree (University of Cambridge, UK) and Kate Heesom (Univesity of Bristol, UK).
Effects of S-acylation of protein distribution Loss of an S-acyl transferase is likely to affect the distribution of acylated proteins. Using a LOPIT (Dunkeley et al., 2004), (Dunkeley et al., 2006) approach we intend to assess the effects of loss of S-acyl transferase activity in Tip1- plants on protein distribution. This will allow us to determine the effects of S-acylation alone and in combination with other membrane anchors on protein subcellular localisation. This is being performed in collaboration with Paul Dupree and Kathryn Lilley (Cambridge, UK)
Effects of TIP1 on Type2 ROP palmitoylation We are currently investigating whether TIP1 is responsible for the S-acylation of ROPs 9, 19 and 11 in collaboration with Shaul Yalovsky (Tel-Aviv, Israel)
TIP1 and S-acylation publications
Multiple roles for protein palmitoylation in plants. Hemsley, P.A., Grierson, C.S. (2008) Trends in Plant Science. 13(6):295-302.
Assaying protein palmitoylation in plants. Hemsley PA, Taylor L, Grierson CS. Plant Methods. (2008) 4:2.
The TIP GROWTH DEFECTIVE1 S-acyl transferase regulates plant cell growth in Arabidopsis. Hemsley PA, Kemp AC & Grierson CS. Plant Cell. (2005) 17(9):2554-63.
TIP1 is required for both tip growth and non-tip growth in Arabidopsis. Ryan, E., Grierson, C.S., Cavell, A., Steer, M., and Dolan, L. New Phytologist. (1998). 138, 49–58
Pollen tube and root-hair tip growth is disrupted in a mutant of Arabidopsis thaliana. Schiefelbein, J., Galway, M., Masucci, J., and Ford, S. Plant Physiol. (1993) 103, 979–985.