Talk:20.109(F13): Difference between revisions
(Removing all content from page) |
No edit summary |
||
| Line 1: | Line 1: | ||
==Setting up the High throughput viability assay== | |||
In the tissue culture room you will stimulate a 96-well plate of cells that was seeded for you last night by the teaching staff. '''The seeding density for this experiment is 31,250 cells/cm<sup>2</sup>.''' | |||
Just like on M2D1, you will have a ''dilution plate'' and an ''experimental plate'' -- this time your experimental plate has cells plated in it! | |||
Your ''dilution'' plate will follow this layout: [[Image:Dilution_plate_M2D6_F13.jpg]] | |||
Here: | |||
*E = Erlotinib | |||
*X = Your inhibitor (LY29004, U0126, or Stattic) | |||
*PC = Positive Control (McCoy's + 1% serum + 1% DMSO) | |||
*EGF = Epidermal growth factor | |||
*M = Media (McCoy's + 1% serum + 12.5 ng/mL EGF) | |||
*M+D = Media + 0.001% DMSO | |||
Your ''experimental'' plate will follow this layout: [[Image:Experimental_plate_M2D6_F13.jpg]] | |||
===Prepare the dilution plate=== | |||
Each well in the ''experimental'' plate will contain 100 μL of total volume. Half of that volume (50 μL) will contain Erlotinib and the other half (50 μL) will contain inhibitor X. <font color = red>The inhibitors will mix '''and be diluted 2x''' when you transfer them from the ''dilution'' plate to the ''experimental'' plate.</font color> Therefore, we will double the highest concentration of each inhibitor when preparing the ''dilution'' plate -- for example, the highest concentration of Erlotinib on our ''experimental plate'' will be 10 μM, so we will double that to 20 μM on our ''dilution'' plate. If this doesn't make sense to you, stop here and ask the teaching staff. | |||
Set-up the ''dilution'' plate as follows (use a multichannel pipette and trough where you can): | |||
#Set-up M and M + D wells: | |||
#*To each M well in the ''dilution'' plate, add 225 μL of McCoy's media + 1% serum + 12.5 ng/mL EGF. | |||
#*To each M+D well in the ''dilution'' plate, add 200 μL of McCoy's media + 1% serum + 12.5 ng/mL EGF + 0.001% DMSO. | |||
#To each well marked E20 or X1, add 275 μL of Erlotinib (20μM) or Inhibitor X stock solution, respectively. | |||
#*LY294002 stock = 40 μM | |||
#*U0126 stock = 20 μM | |||
#*Stattic stock = 40 μM | |||
#Starting with the Erlotinib stock solution, use the multichannel pipette with '''6''' pipette tips attached to move 25 μL from row A to row B by only pressing the pipette plunger to the first stop. | |||
#Mix your solutions by pipetting up and down 4-5 times -- but always stop at the first stop to avoid adding air bubbles! | |||
#Using the same pipette tips, move 25 μL from row B to row C. Repeat your mixing and serial dilutions until row E. | |||
#*'''Do not transfer inhibitor to row F.''' | |||
#Next, repeat the same protocol diluting the Inhibitor X stock solution across columns 8-11. | |||
#*'''Do not transfer inhibitor to column 12.''' | |||
#To wells G1 and H1, add 250 μL of PC (Media + 1% DMSO) | |||
#Now, set-up your EGF dilutions. This must be done in eppendorf tubes because the volume is too large. | |||
#*Label 5 eppendorf tubes with: | |||
#**50 ng/mL | |||
#**25 ng/mL | |||
#**12.5 ng/mL | |||
#**6.25 ng/mL | |||
#**0 ng/mL | |||
#*Add 500 μL of McCoy's media + 1% serum to each eppendorf tube. | |||
#*Pick up an aliqout of 500 μL of 100 ng/mL EGF (15.8 nM) from the center bench. | |||
#*Serial dilute two-fold (remove 500 μL from the highest concentration and put into the 50 ng/mL tube) and mix well. | |||
#*Repeat until you've diluted to 6.25 ng/mL EGF. '''Do not add EGF to the tube labeled 0 ng/mL.''' | |||
#*Finally, transfer 250 μL of each EGF solution to the appropriate well in the ''dilution'' plate. | |||
Notes: | |||
#We are adding 12.5 ng/mL of EGF to each well with inhibitor so that we can control how much we activate the EGFR signaling pathway. And, as you will likely see from the EGF dose-response curve (in green), using too much EGF will result in cell death by itself! Therefore, a dose of 12.5 ng/mL EGF has been empirically determined to stimulate cell growth in the SKOV3 cells prior to our experiment. | |||
#We will add 1% serum to our media because the cells will die due to lack of serum components after 48 hours and we are interested in quantifying the efficacy of the inhibitors, not death due to lack of nutrients. Unfortunately, EGF alone will not sustain these cells. | |||
#To the wells without inhibitor we are adding 0.001% DMSO because, similar to the inhibitor you used in Module 1, our inhibitors are resuspended in DMSO. | |||
#There are no cells in wells G1 and H1. These wells will serve to measure the background of the viability assay on M2D7. | |||
===Prepare the experimental plate=== | |||
Finally, let's prepare our ''experimental'' plate. You should read through this protocol and remember what you did on M2D1 -- this is important because the first thing you will do is remove almost all the media from on top of your cells. You don't want to leave them dry for long because they will die. Recall your ''experimental'' plate from M2D1 before moving on. [[Image:Close_up_experimental_plate.jpg|center|400px]] | |||
#Aspirate ''almost'' all of the media from the cells in your experimental plate using the vacuum aspirator. You do not need to change aspirating tips or rinse with ethanol between each step. Do this step quickly, but with care not to touch the cells! | |||
#Using the multi-channel pipette with '''6''' pipette tips attached, add 50 μL from column 12 from your ''dilution'' plate to columns 6 and 12 of a new 96-well plate (your ''experimental'' plate). | |||
#*Note: We deliberately started at the lowest drug concentration (zero!) because that will allow you to re-use the same pipette tips for the next steps. | |||
#Add 50 μL from column 11 of the ''dilution'' plate to columns 5 and 11 of the ''experiment'' plate. | |||
#Add 50 μL from column 10 of the ''dilution'' plate to columns 4 and 10 of the ''experiment'' plate. | |||
#Add 50 μL from column 9 of the ''dilution'' plate to columns 3 and 9 of the ''experiment'' plate. | |||
#Add 50 μL from column 8 of the ''dilution'' plate to columns 2 and 8 of the ''experiment'' plate. | |||
#Add 50 μL from column 7 of the ''dilution'' plate to columns 1 and 7 of the ''experiment'' plate. | |||
#Add '''6''' new pipette tips to your multi-channel pipetteman. | |||
#Now add the Erlotinib -- begin by pipetting the drug contents (50 μL) of row F from the ''dilution'' plate into row F of the ''experiment'' plate. | |||
#*Note: You will need to perform two pipetting steps to fill all of row F (columns 1-6 and then 7-12). | |||
#*'''Change your pipette tips with each new addition so that you do not contaminate your Erlotinib stock with Inhibitor X''' | |||
#Continue by adding 50 μL from row E of the ''dilution'' plate to row E of the ''experiment'' plate. | |||
#Fill the remaining rows with increasing Erlotinib. | |||
#Finally, transfer the 100 μL of the media containing the PC and EGF dose-response curve to your ''experimental'' plate. You may also use a multi-channel pipette for this step and you do not need to change tips between pipetting steps. | |||
Once you are finished, return your ''experimental'' plate to the incubator and aspirate the remaining contents of your ''dilution'' plate. Clean up the tissue culture hood and you are finished with tissue culture for the semester! | |||
Latest revision as of 10:26, 30 October 2013
Setting up the High throughput viability assay
In the tissue culture room you will stimulate a 96-well plate of cells that was seeded for you last night by the teaching staff. The seeding density for this experiment is 31,250 cells/cm2.
Just like on M2D1, you will have a dilution plate and an experimental plate -- this time your experimental plate has cells plated in it!
Your dilution plate will follow this layout: 
Here:
- E = Erlotinib
- X = Your inhibitor (LY29004, U0126, or Stattic)
- PC = Positive Control (McCoy's + 1% serum + 1% DMSO)
- EGF = Epidermal growth factor
- M = Media (McCoy's + 1% serum + 12.5 ng/mL EGF)
- M+D = Media + 0.001% DMSO
Your experimental plate will follow this layout: 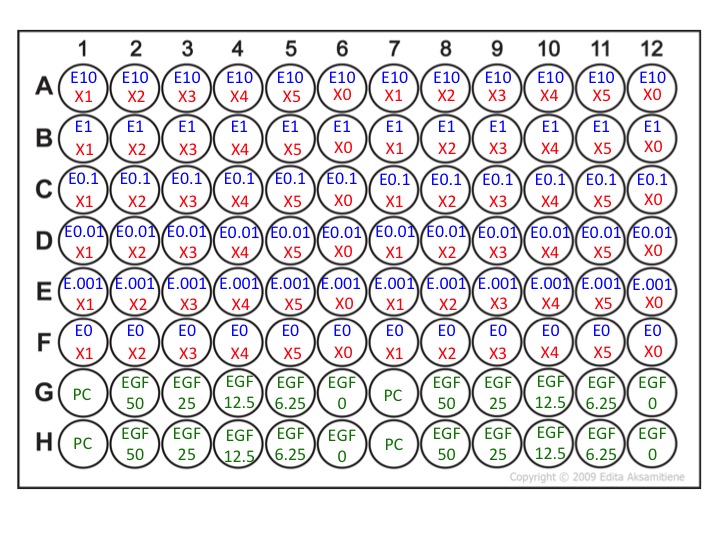
Prepare the dilution plate
Each well in the experimental plate will contain 100 μL of total volume. Half of that volume (50 μL) will contain Erlotinib and the other half (50 μL) will contain inhibitor X. The inhibitors will mix and be diluted 2x when you transfer them from the dilution plate to the experimental plate. Therefore, we will double the highest concentration of each inhibitor when preparing the dilution plate -- for example, the highest concentration of Erlotinib on our experimental plate will be 10 μM, so we will double that to 20 μM on our dilution plate. If this doesn't make sense to you, stop here and ask the teaching staff.
Set-up the dilution plate as follows (use a multichannel pipette and trough where you can):
- Set-up M and M + D wells:
- To each M well in the dilution plate, add 225 μL of McCoy's media + 1% serum + 12.5 ng/mL EGF.
- To each M+D well in the dilution plate, add 200 μL of McCoy's media + 1% serum + 12.5 ng/mL EGF + 0.001% DMSO.
- To each well marked E20 or X1, add 275 μL of Erlotinib (20μM) or Inhibitor X stock solution, respectively.
- LY294002 stock = 40 μM
- U0126 stock = 20 μM
- Stattic stock = 40 μM
- Starting with the Erlotinib stock solution, use the multichannel pipette with 6 pipette tips attached to move 25 μL from row A to row B by only pressing the pipette plunger to the first stop.
- Mix your solutions by pipetting up and down 4-5 times -- but always stop at the first stop to avoid adding air bubbles!
- Using the same pipette tips, move 25 μL from row B to row C. Repeat your mixing and serial dilutions until row E.
- Do not transfer inhibitor to row F.
- Next, repeat the same protocol diluting the Inhibitor X stock solution across columns 8-11.
- Do not transfer inhibitor to column 12.
- To wells G1 and H1, add 250 μL of PC (Media + 1% DMSO)
- Now, set-up your EGF dilutions. This must be done in eppendorf tubes because the volume is too large.
- Label 5 eppendorf tubes with:
- 50 ng/mL
- 25 ng/mL
- 12.5 ng/mL
- 6.25 ng/mL
- 0 ng/mL
- Add 500 μL of McCoy's media + 1% serum to each eppendorf tube.
- Pick up an aliqout of 500 μL of 100 ng/mL EGF (15.8 nM) from the center bench.
- Serial dilute two-fold (remove 500 μL from the highest concentration and put into the 50 ng/mL tube) and mix well.
- Repeat until you've diluted to 6.25 ng/mL EGF. Do not add EGF to the tube labeled 0 ng/mL.
- Finally, transfer 250 μL of each EGF solution to the appropriate well in the dilution plate.
- Label 5 eppendorf tubes with:
Notes:
- We are adding 12.5 ng/mL of EGF to each well with inhibitor so that we can control how much we activate the EGFR signaling pathway. And, as you will likely see from the EGF dose-response curve (in green), using too much EGF will result in cell death by itself! Therefore, a dose of 12.5 ng/mL EGF has been empirically determined to stimulate cell growth in the SKOV3 cells prior to our experiment.
- We will add 1% serum to our media because the cells will die due to lack of serum components after 48 hours and we are interested in quantifying the efficacy of the inhibitors, not death due to lack of nutrients. Unfortunately, EGF alone will not sustain these cells.
- To the wells without inhibitor we are adding 0.001% DMSO because, similar to the inhibitor you used in Module 1, our inhibitors are resuspended in DMSO.
- There are no cells in wells G1 and H1. These wells will serve to measure the background of the viability assay on M2D7.
Prepare the experimental plate
Finally, let's prepare our experimental plate. You should read through this protocol and remember what you did on M2D1 -- this is important because the first thing you will do is remove almost all the media from on top of your cells. You don't want to leave them dry for long because they will die. Recall your experimental plate from M2D1 before moving on.

- Aspirate almost all of the media from the cells in your experimental plate using the vacuum aspirator. You do not need to change aspirating tips or rinse with ethanol between each step. Do this step quickly, but with care not to touch the cells!
- Using the multi-channel pipette with 6 pipette tips attached, add 50 μL from column 12 from your dilution plate to columns 6 and 12 of a new 96-well plate (your experimental plate).
- Note: We deliberately started at the lowest drug concentration (zero!) because that will allow you to re-use the same pipette tips for the next steps.
- Add 50 μL from column 11 of the dilution plate to columns 5 and 11 of the experiment plate.
- Add 50 μL from column 10 of the dilution plate to columns 4 and 10 of the experiment plate.
- Add 50 μL from column 9 of the dilution plate to columns 3 and 9 of the experiment plate.
- Add 50 μL from column 8 of the dilution plate to columns 2 and 8 of the experiment plate.
- Add 50 μL from column 7 of the dilution plate to columns 1 and 7 of the experiment plate.
- Add 6 new pipette tips to your multi-channel pipetteman.
- Now add the Erlotinib -- begin by pipetting the drug contents (50 μL) of row F from the dilution plate into row F of the experiment plate.
- Note: You will need to perform two pipetting steps to fill all of row F (columns 1-6 and then 7-12).
- Change your pipette tips with each new addition so that you do not contaminate your Erlotinib stock with Inhibitor X
- Continue by adding 50 μL from row E of the dilution plate to row E of the experiment plate.
- Fill the remaining rows with increasing Erlotinib.
- Finally, transfer the 100 μL of the media containing the PC and EGF dose-response curve to your experimental plate. You may also use a multi-channel pipette for this step and you do not need to change tips between pipetting steps.
Once you are finished, return your experimental plate to the incubator and aspirate the remaining contents of your dilution plate. Clean up the tissue culture hood and you are finished with tissue culture for the semester!