Talk:20.109(S08):Protein-level analysis (Day6): Difference between revisions
Eva Klinman (talk | contribs) |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 397: | Line 397: | ||
<h3>RT-PCR analysis</h3> | <h3>RT-PCR analysis</h3> | ||
Following RNA isolation of our three samples, we performed RT-PCR using about 270 ng of RNA for each reaction with collagen I (CN I) and collagen II (CN II) primers. In the visualization of our RT-PCR fragments on agarose gel with CN I and CN II controls, the band intensities were normalized by using the same band area and subtracting off backround intensity, and are indicated in Table 1. Our data indicated that the weighted 3D sample expressed more CN II than unweighted 3D and on comparable levels with the CN II control. For CN I, unweighted 3D control had the most expression than all other samples. With regard to the CN II:CN I ratio, the 3D weighted sample had a much higher ratio (21.7) than all other samples including the collagen controls (5.52). As predicted, this demonstrates that the 3D weighted cells exhibit chondrocyte phenotype, while the 2D and 3D controls do not. For CN I, the control and 3D control had similar intensities and similar amounts of DNA (area of band on gel). For CN II, control and 3D weighted have similar intensities and amounts of DNA. Yet for the 2D sample, it was about half as intense and the band area was about 1/2 to 1/3 of the other two samples. These results confirmed our hypothesis that applying mechanical stress promotes chondrocyte phenotype. | Following RNA isolation of our three samples, we performed RT-PCR using about 270 ng of RNA for each reaction with collagen I (CN I) and collagen II (CN II) primers. The 2D, 3D control, and 3D weight had RNA concentrations of 53.6, 22.0, and 18.4 ug/mL, respectively. In the visualization of our RT-PCR fragments on agarose gel with CN I and CN II controls, the band intensities were normalized by using the same band area and subtracting off backround intensity, and are indicated in Table 1. On the agarose gel, our 3D-1 was 3D control and 3D-2 was 3D culture under mechanical stress (weight). Our data indicated that the weighted 3D sample expressed more CN II than unweighted 3D and on comparable levels with the CN II control. For CN I, unweighted 3D control had the most expression than all other samples. With regard to the CN II:CN I ratio, the 3D weighted sample had a much higher ratio (21.7) than all other samples including the collagen controls (5.52). As predicted, this demonstrates that the 3D weighted cells exhibit chondrocyte phenotype, while the 2D and 3D controls do not. For CN I, the control and 3D control had similar intensities and similar amounts of DNA (area of band on gel). For CN II, control and 3D weighted have similar intensities and amounts of DNA. Yet for the 2D sample, it was about half as intense and the band area was about 1/2 to 1/3 of the other two samples. These results confirmed our hypothesis that applying mechanical stress promotes chondrocyte phenotype. | ||
'''Table 1.''' Band intensities and areas on agarose gel electrophoresis of RT-PCR fragments and CN I and CN II controls. Intensity values are normalized by band area (of 0.002 using ImageJ from NIH) and area values reflect the total area of the band. | '''Table 1.''' Band intensities and areas on agarose gel electrophoresis of RT-PCR fragments and CN I and CN II controls. Intensity values are normalized by band area (of 0.002 using ImageJ from NIH) and area values reflect the total area of the band. | ||
| Line 453: | Line 453: | ||
<h3>ELISA results</h3> | <h3>ELISA results</h3> | ||
All of our collagen II sample absorbance values fell out of the low range of our assay and were barely measurable. For collagen I, only the 3D control supernatant, 3D control cells, and 2D cells had positive absorbance values after subtracting off background absorbance. Using a calibration curve derived from collagen I and II standards and subtracting background absorbance values off of all data, we calculated the following CN II: CN I ratios, which reflects the relative degree of chondrocyte-like phenotype. We believe these data give little valuable information because our collagen II levels were so low. The only conclusions we could draw are that the 3D control had were more chondrocyte-like than 3D weighted, and the 2D was even more chondrocyte-like than both 3D cultures. This contradicts our cell morphology and transcript analysis, where we found that the cells in 3D culture under mechanical stress exhibited the most chondrocyte-like phenotype while the 3D control and 2D cells were relatively de-differentiated into fibroblast-like cells. | |||
<div align="center"> | |||
{| border="1" | |||
! Sample | |||
! CN II: CN I supernatant | |||
! CN II: CN I cell | |||
! CN II: CN I supernatant | |||
! CN II: CN I cell | |||
|- | |||
| 2D | |||
| 0.0104 | |||
| 0.0038 | |||
| 0.0050 | |||
| 0.0024 | |||
|- | |||
| 3D control | |||
| 0.0074 | |||
| 0.0075 | |||
| 0.0039 | |||
| 0.0041 | |||
|- | |||
| 3D weight | |||
| 0.0028 | |||
| 0.0037 | |||
| 0.0014 | |||
| 0.0018 | |||
|- | |||
|} | |||
</div> | |||
==Wednesday/Friday Yellow== | ==Wednesday/Friday Yellow== | ||
'''Background''' | |||
We wanted to test how the strength of the alginate beads would affect cell growth and dedifferentiation. Thus, for our experiment we decided to use calcium and magnesium to form two different alginate bead scaffoldings. The concentration we used for magnesium was not correct and thus it did not form alginate beads. We then went ahead to test our calcium sample, but they were misplaced. Thus we ended up using another groups samples. They added a calcium chelating agent to one of there samples, which they thought would reduce the mechanical strength of the alginate scaffolding by stopping the G block interactions, which would cause more chondrocytes to de-differentiate into fibroblasts. There other samples acted as controls. Only 100,000 cells were initially seeded into the 2D sample and 1.5 million cells were seeded into the 3D samples. The chelating agent was added to 3D-sample 2 at a concentration of 1%. | |||
'''Cell Recovery & Live/Dead Fluorescence Assay''' | |||
Our cell counts were very low on day 3 for our 3D sample. We determined our 2D sample had around 900,000 cells/mL that were alive and around 11,000 cells/mL that were dead. On the other hand, our 3D sample only had around 30,000 cells/mL that were alive and around 11,000 cells/mL that were dead. The cells in our 3D sample had a round shape and were clustered together, while the cells in our 2D sample were elongated which matched a fibroblast morphology. Since we had low cell numbers, we took the maximum amount of each sample for our Live/Dead fluorescence assay. | |||
'''RT-PCR & Agarose Gel''' | |||
We started using another groups sample at this point and used there data for the RNA content. Below is the amount of RNA they used in their RT-PCR samples. | |||
We measured the collagen II to collagen I ratio of our samples by examining the RNA content as well as collagen expression of our samples. With the first half of our samples, we extracted all RNA for RT-PCR. The following is the amount of RNA we used in RT-PCR for each sample: | |||
<div align="center"> | |||
{| border="1" | |||
! Sample | |||
! RNA Concentration (ug/mL) | |||
! Max RNA (ng in 30 uL) | |||
|- | |||
| 2D | |||
| 104 | |||
| 200 | |||
|- | |||
| 3D Sample 2 | |||
| 18 | |||
| 200 | |||
|} | |||
</div> | |||
We used 1/10th of the RNA concentration their group used in order to transcribe the collagen RNA to cDNA and then amplified this cDNA through PCR. | |||
Upon running our RT-PCR products on an agarose gel we only had bands for the 3D collagen 1 and 2D collagen 2, so we could not really compare the samples. Using ImageJ, to analyze the intensity of the bands and found the following ratios: | |||
<div align="center"> | |||
{| border="1" | |||
! Sample | |||
! CN-II : CN-I Mean Intensity | |||
|- | |||
| 2D collagen 2 | |||
| 40.459 | |||
|- | |||
| 3D collagen 1 | |||
| 68.550 | |||
|} | |||
</div> | |||
Our bands were really close in size so we were able to determine the amount of DNA in each band relative to one another directly from the intensity. It appears that there is around 1.7X as much DNA in our 3D sample as our 2D sample. Which shows that if you just look at collagen types, it appears there is a high collagen I:II ratio. Since the samples came from 3D and 2D it is hard to compare them to conclude anything about chondrocyte phenotype development. | |||
'''ELISA''' | |||
We ran ELISA as a secondary method to measure the collagen I:II ratio. Our collagen one and our collagen two data was higher than the empty wells but below our blank. Thus, our sample data was out of range of the standard curve, so we couldn’t draw any real conclusions from the data. Although we couldn’t draw any conclusions, some absorbance was still seen. This means that there might of have been some collagen I and II present, but further experiments would need to be run. | |||
==Wednesday/Friday Green== | ==Wednesday/Friday Green== | ||
| Line 539: | Line 621: | ||
'''Cell Recovery & Live/Dead Fluorescence Assay''' | '''Cell Recovery & Live/Dead Fluorescence Assay''' | ||
Upon trypan exclusion on day 3, we found cell counts to be extremely low. We determined that our 2D sample had around 500,000 cells while each of our 3D samples had only around 11,000 cells. Preliminary microscopy showed no difference in the morphology of our 3D samples. However, we noticed that most of the cells in our 2D sample had an oblong shape consistent with fibroblast morphology. Because of our low cell count, we took the maximum amount of each sample (1.5mL) for our Live/Dead fluorescence assay. Despite this, cell counts were too low for us to observe many live or dead cells. | Upon trypan exclusion on day 3, we found cell counts to be extremely low. We determined that our 2D sample had around 500,000 cells/mL while each of our 3D samples had only around 11,000 cells/mL. Preliminary microscopy showed no difference in the morphology of our 3D samples. However, we noticed that most of the cells in our 2D sample had an oblong shape consistent with fibroblast morphology. Because of our low cell count, we took the maximum amount of each sample (1.5mL) for our Live/Dead fluorescence assay. Despite this, cell counts were too low for us to observe many live or dead cells. | ||
'''RT-PCR & Agarose Gel''' | '''RT-PCR & Agarose Gel''' | ||
We measured the collagen II to collagen I ratio of our samples by examining the RNA content as well as collagen expression of our samples. With the first half of our samples, we extracted all RNA for RT-PCR. Using collagen specific primers, we reverse transcribed the collagen RNA to cDNA and then amplified this cDNA through PCR. Upon running our RT-PCR products on an agarose gel we found that our Col II:Col I ratio was high in all of our samples. Using ImageJ, we analyzed the intensity of the agarose gel bands and found the following ratios: | We measured the collagen II to collagen I ratio of our samples by examining the RNA content as well as collagen expression of our samples. With the first half of our samples, we extracted all RNA for RT-PCR. The following is the amount of RNA we used in RT-PCR for each sample: | ||
<div align="center"> | |||
{| border="1" | |||
! Sample | |||
! RNA Concentration (ug/mL) | |||
! Max RNA (ng in 30 uL) | |||
|- | |||
| 2D | |||
| 104 | |||
| 200 | |||
|- | |||
| 3D Sample 1 | |||
| N/A | |||
| N/A | |||
|- | |||
| 3D Sample 2 | |||
| 18 | |||
| 200 | |||
|} | |||
</div> | |||
Although we did not detect any RNA in 3D-Sample 1, we suspected that there might be some RNA present. We decided to proceed with the max volume (20 uL) for RT-PCR of 3D-Sample 1. | |||
Using collagen specific primers, we reverse transcribed the collagen RNA to cDNA and then amplified this cDNA through PCR. Upon running our RT-PCR products on an agarose gel we found that our Col II:Col I ratio was high in all of our samples. Using ImageJ, we analyzed the intensity of the agarose gel bands and found the following ratios: | |||
<div align="center"> | <div align="center"> | ||
| Line 561: | Line 667: | ||
</div> | </div> | ||
'' | Although our transcript-level analysis shows the Col II:I ratio of 3D-Sample I to be higher than that of 3D-Sample II, the difference is not significant enough to conclude whether chondrocyte phenotype development was hindered by the calcium chelator. Furthermore, since we are unsure of the initial amount of RNA present in 3D-Sample 1, it is difficult to compare the ratios accurately. | ||
'''ELISA''' | |||
We performed ELISA in order to analyze the Col II : Col I ratio of our samples. Following is the initial volume of cells in 1 ml which we started with. | |||
<div align="center"> | |||
{| border="1" | |||
! Sample | |||
! # Cells | |||
|- | |||
| 2D | |||
| 922,222 cells | |||
|- | |||
| 3D Sample 1 | |||
| 33,333 cells | |||
|- | |||
| 3D Sample 2 | |||
| 44,444 | |||
|} | |||
</div> | |||
The results of our ELISA were overall unreliable and generally incomparable to our transcript-level analysis. We noted a few mistakes in protocol which reduced the validity of our data. First, we added primary antibody to our standards, but forgot to add the primary antibody to our samples the day we were supposed to. Thus, our standards sat with antibody and then block buffer, however our samples were sitting in dry wells overnight. We attempted to correct this mistake by adding primary antibody the next day, but knew our results were going to be questionable. Second, by the time we were ready to add secondary antibody to our plates, block buffer was out of stock. As such we diluted our secondary antibody in PBS rather than block buffer. Also, because we developed our plates 1 day after other groups, the development buffer had been left out at room temperature for > 24hrs. These mistakes in protocol coupled with our low initial sample recovery contributed to the poor results of our ELISA. | |||
Though we did not expect to obtain significant results, we nevertheless ran our ELISA. Our Collagen I sample was left to develop overnight. As such, we observed a small amount of absorption in these wells. Upon comparison to the standard Collagen I curve, we found this data to be unreliable since the highest OD value was still lower than the lowest value on our standard curve. In other words, our sample data was out of range of our standard curve. Though we cannot extract concrete information about the Collagen I content of our samples from this data, the fact that there was any absorbance at all suggests that Collagen I might be present. We would however need to redo the experiment (possibly modified) to conclusively state this. | |||
Our Collagen II samples showed no absorbance at 420 nm. Perhaps if these samples had been allowed to develop overnight some activity would have been observed. Since we did not obtain any Collagen II data from ELISA, we cannot make any comment on the ratio of Col II: Col I based on this assay. | |||
==Wednesday/Friday Pink== | ==Wednesday/Friday Pink== | ||
Latest revision as of 15:04, 14 May 2008
Tuesday/Thursday Red
We chose to vary the viscosity of our alginate beads. We used 500,000 cells for our 2-D cultures and a density of 5,000,000 cells/mL in our 3-D samples. For our low-viscosity 3-D sample, we used Sigma-Aldrich alginate, while for our high-viscosity sample, we used FMC 10/60; both alginates were used at 2%. In addition, we added ascorbate to our 3-D samples only to enhance the contrast between our 2-D and 3-D culture results. Our light microscope cell count showed very few cells in our 3-D samples; however, a microscope examination of our alginate beads showed extensive cell populations. This discrepancy was possibly due to errors made in the cell isolation process. As a result, our cell viability assay was not very informative, since so few cells were present for analysis. To analyze the differentiation state of our cells, we performed RT-PCR on our cells to isolate cDNA for collagens I and II. RNA extraction was very successful for all of our samples, especially the 3D-High sample. Analysis of the cDNA gel showed a sizeable presence of cDNA in each sample. The 2D sample yielded the highest Collagen II : Collagen I cDNA ratio, followed closely by our 3D-High sample. Our 3D-Low sample yielded the least cDNA, and also had almost a 1:1 ratio of Collage II : I. These transcript results seem to indicate that our cells were relatively healthy and maintained chondrocyte differentiation in 2D culture and high viscosity alginate, but were not so in low viscosity alginate. Unfortunately, our ELISA data yielded the exact opposite results. Only our 3D-Low sample yielded significant amounts of Collagen II, but all samples produced a sizeable amount of collagen I. This would suggest that the low viscosity 3D alginate is best for maintaining chondrocyte differentiation. The extreme discrepancy in our results is most likely due to imprecise execution of the ELISA protocols: using our standards curve, our fitted data gave significantly negative (less than 0) collagen II concentrations for most of our samples.
ELISA Results
| Collagen II | Collagen I | Ratio | |
|---|---|---|---|
| 2D - Sup | 0 | 541.25 | 0 |
| 2D - Cells | 0 | 671.11 | 0 |
| 3D Lo - Sup | 0 | 655.16 | 0 |
| 3D Lo - Cells | 140.69 | 625.66 | 0.22 |
| 3D Hi - Sup | 0 | 567.74 | 0 |
| 3D Hi - Cells | 0 | 616.19 | 0 |
| BLANK | 0 | 520.19 | 0 |
| 0 | 500.93 | 0 |
| Cell count per mL | |
|---|---|
| 2D | 233,333 |
| 3D Low Viscosity | 1,077,777 |
| 3D High Viscosity | 222,222 |
| RNA Concentration (ug/mL) | |
|---|---|
| 2D | 52.8 |
| 3D Low Viscosity | 54.0 |
| 3D High Viscosity | 135.2 |
Cell volumes for ELISA
| Volume Used(uL) | |
|---|---|
| 2D | 1000 |
| 3D Low Viscosity | 185.5 |
| 3D High Viscosity | 1000 |
| RT-PCR Sample | Contents | |
|---|---|---|
| 3D Culture 1 | Low-viscosity alginates | |
| 3D Culture 2 | High-viscosity alginates |
We used the standard 200ng of RNA per sample for the RT-PCR.
Tuesday/Thursday Yellow
In our experiments we added Collagen II to one of our 3D 1% alginate samples. Our other 3D and 2D samples were cultured normally. Our hypothesis was that the presence of collagen II would reduce the amount of dedifferentiation of the chondrocytes to fibroblasts. When we observed the cells after they were grown for a week, the morphology of the cells in our 3D cultures retained a round phenotype, suggestive of a chondrocyte phenotype. However, the cells in our 2D cultures appeared more spread out, suggesting dedifferentiation info fibroblasts. For each of our cultures, we recovered about 100,000 cells/mL but recovered slightly more (about 155,000 cells/mL) for the 3D culture with collagen. We noticed that the cells in the 3D cultures were very small, but rounded, and we found it difficult to distinguish between simply debris or a very small cell. However, few cells, live or dead, were observed under microscopy in the LIVE/DEAD assay. This may be due to the fact that it was difficult for us to observe cell pellets before aspirating our supernantants and we could have lost cells at this step in the LIVE/DEAD assay. Despite our concerns with having low cell yield and lack of results for the LIVE/DEAD assay, isolation of the RNA for our RT-PCR reactions showed mRNA concentrations of 2D: 6.8ug/mL, 3D+coll: 14 ug/mL, and 3D+none: 8.4 ug/mL. We split our sample for RT-PCR and ELISA, using 1 mL for each. We used 100 ng of RNA for each PCR reaction. We ran the PCR fragments from our RT-PCR on an agarose gel with the intention of comparing the relative intensities of Collagen I and Collagen II cDNA. The samples were run as the protocol described, with 3D-1 corresponding to none and 3D-2 corresponding to with collagen added. Unfortunately, faint bands in many of the lanes could not be photographed and therefore full image analysis of the gel could not be performed. Even so, our image analysis of our 2D sample surprisingly suggested that our 2D samples produced more collagen II than collagen I despite their previously observed fibrobast phenotype. No 3D collagen ratios could be calculated by detection by the computer since no 3D bands were visible on the photo. In general, our collagen II bands, though faint, were stronger than the collagen I bands (many of the collagen I bands were completely missing all together). Our calculated 2D collagen II to collagen II ratio was 1.30, which we didn't expect as we believed that the 2D culture would promote dedifferentiation of chondrocytes. After performing ELISA, we calculated standard curves. After removing outliers for our standard curve data, we were able to calculate measurable quantities of both collagen I (both initial and overnight) and II. All collagen II/I(overnight) ratios were ~.06, except the 3D collagen supernatant which was ~.13. One possible explanation for the large difference between our calculated ratios by transcript analysis and ELISA analysis may have been due to cross-reactivity of our collagen I primary antibodies. This would explain the much higher collagen I concentration obtained through ELISA analysis.
Tuesday/Thursday Green
In our experiment, we chose to test the effect of cell density on cell viability in 1% 3D Sigma alginate scaffolds. Our respective densities were 5x10^6/ml (3D1) and 20x10^6 cells/ml (3D2), versus our 2D T25 sample of 2.5x10^5 cells. Cell counting for the 3D1 sample revealed 4 times as many dead cells for a concentration of 2222 cells/ml, whereas the 3D2 sample revealed approximately 2 times as many live cells per dead cell for a cell count of 3.56x10^5 cells/ml. The 2D sample had a cell count of approximately 3x10^5 cells/ml. Observation under the light microscope also revealed small cell clusters in the 3D2 beads but not in the 3D1 sample beads. 2D cells were slightly round but were starting to exhibit a fibroblast-like phenotype with projections attached to the plastic.
The RNA was isolated, and the total RNA concentrations were 0.0467 ug/ml for the 2D sample and 4.678 ug/ml for the 3D2 sample. The 3D1 sample had a low cell count and unusable (negative) OD value. Therefore, RT-PCR was not performed on this sample. RT-PCR was performed on 7.5ul (50ng) of 2D RNA and "0.1754ul" (50.25ng) of 3D2 RNA. For our RT-PCR gel, the lanes were as follows: (1) ladder, (2) ColI 2D, (3) ColI 3D2, (4) ColII 2D, (5) ColII 3D2, (6) Col I control, (7) Col II control. Analysis of the gel indicated that the ratio of collagen II to collagen I was 2:1 in general for both 2D and 3D2.
For the ELISA sample prep, we obtained a pellet from 1ml of each sample after cell counting. Our ELISA results, interestingly, indicated that for at least the 3D2 sample, the colII:colI ratio was 1:6. This difference in ratios may be due to the direct measurement of proteins versus measuring expression "indirectly" from the supernatant or cells themselves. All collagen I samples had measurable amounts of protein, but for collagen II, only the 3D2 sample had a non-negative number.
Tuesday/Thursday Blue
For our experiment, we chose to vary the cell density added to the 1% alginate beads. All other conditions were held standard. Additionally we prepared a 2D sample with 1 million cells, in the interest of comparing to other groups who prepared 2D samples with varying cell counts. Our two 3D samples were .5 million cells/mL (3D-1) and 10 million cells/mL (3D-2). The results of our light microscope cell count showed no cell recovery for 3D-1. However, 2D and 3D-2 showed comparable cell counts of 310,000 and 270,000 cells/mL, respectively. In all cases the cell count was lower than expected. This most likely due to poor recovery of cells from media and not cell death, since we did not observe dead cells. Our live-dead assay showed no cells for 2D and 3D-1, which could be due to poor staining, dye bleaching, or, again, poor cell recovery from the media. For 3D-2, we observed one live cell (fluoresced green but not red). We then ran RT-PCR on the cDNA from the samples to test for collagen 1 and collagen 2 production. We first purified RNA from the three samples and obtained concentrations of 2 ug/mL, 40 ug/mL, and 45.6 ug/mL, for samples 2D, 3D-1, and 3D-2, respectively. After running RT-PCR on the RNA samples and analyzing 60 ng RNA total from each on agarose gel (using the standard lane schematic), the gel showed no collagen of either type for our 2D sample. This could be due to any number of problems, from low cell viability rate, to poor purification of mRNAs from the cells, or inefficient reverse transcription of the RNA to form cDNA (perhaps because of issues with the primer), or even problems with the PCR step itself. However, for 3D-1, the RT-PCR gel shows that a small amount of collagen 2 was produced, and that for 3D-2, a small amount of collagen 1 and a relatively large amount of collagen 2 were produced. The presence of collagen 2 in these two samples seems to indicate that the cells mostly retained chondrocytic phenotypes, as it is in this phenotype that higher amounts of collagen 2 are produced. These results could also indicate that growing cells in a 3D alginate setting causes increased production of both collagen types, since our 2D culture did have a significant cell count, and it seems unlikely that the RT-PCR failed to work properly only in both 2D samples. From this analysis, we were only able to determine the CNII:CNI ration for 3D-2 sample, which was approximately 3.25:1. We also ran ELISA as a secondary protein-level assay of the ratio of collagen types; however the standards we used were underdeveloped and we did not run our actual samples, so we were unable to determine any ratios (or really, any data at all) from the ELISA experiment. Overall, we were able to determine that for our high cell density culture, collagen II was produced approximately 3X as much as collagen I, which suggests that this sample retained the chondrocyte phenotype, as it is in this phenotype that collagen II is predominately produced.
Tuesday/Thursday Purple (Violet!)
Experimental Background and Set-Up After researching the various external and internal influences that affect the chondrocyte phenotype of cells, we decided to target the viscosity of our alignate beads along with the Red group. We targeted the G/M ratio of the alginate in order to attempt to influence the structure of the polymer formed. For the 3D sample, we altered the cross-linking of the polymer. In one sample we used Sigma Aldrich alginate, this serves as our control. We then decided to use a high cross-linking subject of FMC Biopolymer (Protanal 10/60) to increase the stiffness of the alginate polymer. It has an increased cross-linking due to its high G/M ratio. We suspect that there will be an increase in the production of collagen I and an increase in the cell dedifferentiation with the high cross-linking samples. The Sigma Aldrich sample has low cross-linking and we hypothesize that there will be an increased production of collagen II. We also added ascorbate to the 3D samples to attempt decrease dedifferentiation where we did not add any to the 2D samples. The lack of ascorbate in the 2D samples will cause the cells to dedifferentiate faster. Overall, we hypothesized that the 3D sample with higher cross-linking factor will have chondrocytes become fibroblasts more quickly. Also, the 2D samples not containing ascorbate will also make fibroblasts faster.
For the 3D samples, we used a density of 5 x 10^6 cells/mL and only 5 x 10^5 cells for our 2D samples. For our low viscosity level assay, we used 1% Sigma ALdrich alignate while for the high-level we used 1% FMC Biopolymer. In addition, we added ascorbate to our 3D samples only to create a contrast between our 2D and 3D culture results, as explained above. The 2D sample was split at first confluence at 1:20 after 5-6 days and split at second confluence at 1:10 after day 10-11.
Cell Observations After one week of growth, we observed the cell count of our three samples. Number of Cells per mL: For 2D Sample:
211, 111
For 3D Sample: Sigma Aldrich:
1,111 with 5 dead cells per 4X4 square
FMC:
133,333 with 2 dead cells per 4X4 square
These results seem strange as we expected our FMC sample to have less alive cells because of its high G/M ratio (stiffness of alignate). This could be due to any number of labratory errors with culturing.
Cell Viability Results On Day 3 of the Module, we prepared harvested cells from out three samples to test for cell viability in a Live/Dead© (Invitrogen) cell count assay. Dead cells appeared to be red due to their uptake of ethidium while the live cells appeared green due to the uptake of SYTO10. Conclusions drawn are that the 2D sample had the most amount of live cells, however, most were in cluster formation, showing that they were not separated well. The 3D samples had around the same viability as each other. The images can be seen on the "Talk" page of Day 3. Below are our results:
2D Sample:
approximately 120 live (green) cells---mostly in clusters ~4 dead (red) cells
3D Sample: Sigma Aldrich:
~4 live cells ~2 dead cells
FMC:
~5 live cells ~3 dead cells
These results are different from what was predicted. We thought that the 2D sample would have the most fibroblasts, however they had the most alive cells. Also, we expected the FMC sample to have less alive cells, however, we cannot really accept these results seeing as we could not see many cells. This could be due to labratory errors or leaving our samples in the light for too long.
Cell Counts for Preparing Cell Lysates We prepared cells to test if the cultured chondrocytes have secreted some collagen protein into the surrounding medium. We created two samples; one to test for protein and one to isolate RNA to later test for collagen message. Cell Counts: 2D Sample:
22 cells --- 244,444 cells/mL
3D Sample: Sigma Aldrich:
143 cells --- 1,588,888 cells/mL (cells were in clumps, round)
FMC:
2 cells --- 22,222 cells/mL
These cell counts make more sense than those previously recorded (above). This is because, after a longer period of time, more cells grew in the Sigma Aldrich alginate than the FMC alginate. This has potential to prove our hypothesis as cells cannot grow on a more tense alginate.
RT-PCR Results
We isolated RNA from the samples to conduct RT-PCR. We desired to perform RT-PCR in order to obtain amplified cDNA that would be later run on an agarose gel to compare collagen ratios. The collagen II:I ratio shows the ratio of cells with chondrocyte phenotype. Unfortunately, we could only see our 2D sample and the DNA ladder. This means that the 3D samples did not appear due to not having the correct amount of DNA and due to labratory errors.
RNA Concentrations:
Sample RNA conc. (ug/mL)
2D 11.2
3D Sigma 540
3D FMC 0
It is interesting to note that there is a higher RNA concentration in the Sigma Aldrich sample than the other two samples. This could be due to the fact that there was more cells in the Sigma sample. We ulitimately ran 168 ng of RNA.
The gel was run with the lanes 1- DNA Ladder and 2- 2D sample collagen I. No 3D FMC (with collagen I or collagen II) samples were run due to not having any resulting RNA concentration.
The results with image analysis:
Sample --- Mean/Min/Max:
Background --- 58.4/28/124
2D Collagen I --- 50.047/9/87
2D Collagen II --- 145/67/196
The intensity ratio of the 2D sample was determined to be 3:1 (collagen II : collagen I). The image can be seen on "Talk" page of Module 3, Day 5. Therefore, the ratio implies that a 3:1 ratio of cells are chondrocytes:fibroblasts (because chondrocytes express more collagen II than fibroblasts).
Indirect ELISA Results We used the protein samples taken after the second cell observation in order to perform indirect ELISA. We desired to perform ELISA to quanitify specific proteins (collagen I and collagen II) in order to determine the chondrocyte phenotype of our samples.
(S = supernatant, C = Cell) Collagen I (y = 13.767x - 0.5706) Collagen II (y = 0.672x - 0.0389)
Our Collagen Ratio Table:
| Sample | Collagen I (in ug/mL) | Collagen II (in ug/mL) | CI : CII ratio |
|---|---|---|---|
| 2D Supernatant | NONE | NONE | NONE |
| 2D cell | 1.21 | 0.0144 | 84.44041 |
| 3D Sigma S. | 1.01 | 0.0207 | 48.98 |
| 3D Sigma C. | 0.86 | 0.0088 | 96.73 |
| 3D FMC S. | 4.32 | 0.0184 | 235.4 |
| 3D FMC C. | 3.21 | 0.0238 | 134.2 |
For ELISA, we took unequal amounts of cells. We took from 2D 1 ml (~250,000 cells), 3D FMC 1 mL (~22,000 cells), and 3D sigma .15 mL (~250,000 cells). As the results show, the FMC collagen I (fibroblasts): collagen II(chondocytes) ratio was the highest, proving that there was a larger amount of fibroblasts than chondrocytes due to the more tense alginate. The 2D sample also had a higher collagen I: collagen II ratio because of the lack of ascorbate (nutrients). The 3D sigma had the lowest ratio (supernatant), meaning that it did not de-differentiate quickly. The 3D sigma cells had a higher ratio than the 2D cells, but this could be due to labratory errors or the lack of protein expression. We would like to believe that our hypothesis was proven correctly, but we would need to perform this experiment again in order to verify the results and also to obtain results when using the RT-PCR/agarose gel assay. Since we only got a bands for the 2D sample, we cannot compare the results.
Overall, we proved our hypothesis that the 3D sigma alignate allowed for higher chondrocyte phenotype due to analyzing our ELISA results.
Tuesday/Thursday Pink
In our experiment we tested 2D plating with rich medium versus 3D plating in poor medium in the presence or absence of an actin inhibitor toxin. Our toxin was solubilized in DMSO, also toxic to cells; the negative control received DMSO without the toxin. Our LIVE/ DEAD Assay yielded no results as our cells were photobleached because we neglected to wrap the samples in tin foil. We recovered many cells from the 2D sample and the 3D sample with toxin. We were unable to amplify the 3D without toxin sample in our RT PCR reaction. The bright bands on our gel were as follows: 2D Collagen II, 2D Collagen I at both 5 and 10 μL loading volumes. Image J analysis of the bands showed that the Collagen II to Collagen I ratio in the 2D sample was ~2.7. ELISA Analysis was performed on the cell lysates, and standard curves were generated with Bovine Serum Albumin (BSA). The results of ELISA yielded a 2D Collagen II to 2D Collagen I ratio of .18.
Day 3:
- 2D Morphology and Density Observations:
- Sample- Round + dense
- 3D Morphology and Density Observations:
- (-)Toxin: Cells were clumped together alginate funky shaped – perhaps created by droplet too quickly.
- (+) Toxin: Cells more homogenous, alginate round.
- 2D Cell Culture Counts:
- 3.77 *10^5 cells/mL
- Viability = 100%
- 3D Cell Culture Counts:
- (+) Toxin: 450 cells/mL, viability ~10%
- (-) Toxin: 900 Cells/mL, viability ~20%.
- Concern: Resuspension for 3D not homogenous.
- LIVE/DEAD Assay:
- Very few cells seen on either. Did not cover with aluminum foil.
- Went back to look at 2D samples, observed many cells.
Day 4:
- Cell Counts:
- 2D: 5.5*10^5 cell/mL, added 0.5 mL protein, rest of solution was used for RNA.
- 3D (+) Toxin: 2*10^5 cells/mL, added 1mL protein, rest of solution was used for RNA.
- 3D(-) Toxin: 1*10^4 cells/mL with lots of specks of dead cells, added 1mL protein, rest of solution was used for RNA.
- Normalized cells concentrations to 3D(+).
- RNA concentrations: used 200ng RNA per reaction for RT-PCR
- 2D: 198 μg/mL. Amount added (per reaction): 1.01 uL
- 3D(-): ~0. Amount added: 0 uL
- 3D(+): 12 μg/mL. Amount added (per reaction): 16.7 uL
- Hypothesis: DMSO kills cells.
Day 5:
| Lane | Content | Volume | Observation |
|---|---|---|---|
| 1 | Ladder | 11 uL | strong |
| 2 | 2D Collagen I 5uL | 18 uL | dim, low on gel |
| 3 | 3D Collagen I 5uL | 18 uL | not visible |
| 4 | 2D Collagen II 5uL | 18 uL | strong, middle of gel |
| 5 | 3D Collagen II 5uL | 18 uL | not visible |
| 6 | 2D Collagen I 10uL | 18 uL | strong, low on gel |
| 7 | 3D Collagen I 10uL | 18 uL | not visible |
| 8 | 2D Collagen II 10uL | 18 uL | very bright, middle of gel |
| 9 | 3D Collagen II 5uL | 18 uL | not visible |
| 10 | Control I 5uL | 18 uL | not visible |
| 11 | Control II 5uL | 18 uL | dim, low on gel |
| Lane | Area | Mean | Min | Max |
|---|---|---|---|---|
| Background | 0.043 | 28.7 | 6 | 87 |
| 4 | 0.031 | 37.427 | 8 | 127 |
| 6 | 0.026 | 34.9 | 7 | 78 |
| 8 | 0.038 | 94.8 | 18 | 206 |
Day 6/7: Elisa Protein Analysis
- Collagen I plate had bubbles, so we did not analyze that data and looked at the Collagen I O/N data instead.
- All the samples of Collagen II and Collagen I O/N were able to get results with protein concentrations in ng/mL
Wednesday/Friday Red
We, along with the pink group, are testing the effects of mechanical stress on the maintenance of chondrocyte phenotype. We are comparing our 2D and 3D control samples to a set of cells in 3D culture that are under constant mechanical stress in the form of two washers (49.2 g total) placed on top of our petri dish apparatus (Figure 1). Our 3D control chondrocyte-seeded beads were placed in the apparatus without a filter and any weights.
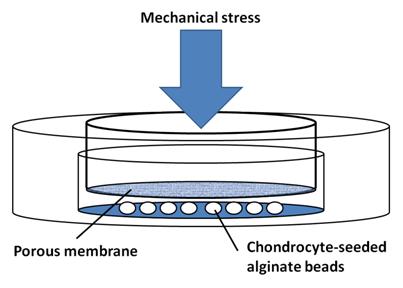
We seeded our 1% Sigma alginate beads with a cell density of 5 million/mL and used normal medium. For 2D culture, we used 600,000 cells per flask (total 1.2 million) in normal medium, split them every 4 days at 1:10.
Cell Morphology
As expected, the 2D cells appeared de-differentiated and exhibited fibroblast phenotype (strand-like) under the light microscope while our 3D control cells maintained a chondrocyte phenotype (round). We were unable to observe our 3D weighted cells because the beads were covered by the filter.
Cell Viability
In terms of cell count, we had very few cells in both of our 3D cultures. There were approximately 455,555 cells/mL in 2D, 33,333 cells/mL in 3D control, and 11,111 cells/mL in 3D weighted. In both 3D cultures, the lower counts may reflect the limited exposure to nutrients and oxygen to cells in the alginate beads. Moreover, 3D culture under mechanical stress had even fewer cells possibly because the filter further limited the flow of nutrients and oxygen to cells. In the live/dead fluorescence assay, the cells in 2D and both 3D cultures were similar in the low number of live and of dead cells, perhaps due to low recovery and incomplete staining.
RT-PCR analysis
Following RNA isolation of our three samples, we performed RT-PCR using about 270 ng of RNA for each reaction with collagen I (CN I) and collagen II (CN II) primers. The 2D, 3D control, and 3D weight had RNA concentrations of 53.6, 22.0, and 18.4 ug/mL, respectively. In the visualization of our RT-PCR fragments on agarose gel with CN I and CN II controls, the band intensities were normalized by using the same band area and subtracting off backround intensity, and are indicated in Table 1. On the agarose gel, our 3D-1 was 3D control and 3D-2 was 3D culture under mechanical stress (weight). Our data indicated that the weighted 3D sample expressed more CN II than unweighted 3D and on comparable levels with the CN II control. For CN I, unweighted 3D control had the most expression than all other samples. With regard to the CN II:CN I ratio, the 3D weighted sample had a much higher ratio (21.7) than all other samples including the collagen controls (5.52). As predicted, this demonstrates that the 3D weighted cells exhibit chondrocyte phenotype, while the 2D and 3D controls do not. For CN I, the control and 3D control had similar intensities and similar amounts of DNA (area of band on gel). For CN II, control and 3D weighted have similar intensities and amounts of DNA. Yet for the 2D sample, it was about half as intense and the band area was about 1/2 to 1/3 of the other two samples. These results confirmed our hypothesis that applying mechanical stress promotes chondrocyte phenotype.
Table 1. Band intensities and areas on agarose gel electrophoresis of RT-PCR fragments and CN I and CN II controls. Intensity values are normalized by band area (of 0.002 using ImageJ from NIH) and area values reflect the total area of the band.
| Collagen | Sample | Intensity | Area |
|---|---|---|---|
| I | Control | 29.3 | 0.007 |
| I | 2D | ~0 (band only visible by eye) | ~0 (band only visible by eye) |
| I | 3D control | 47.9 | 0.01 |
| I | 3D weight | 7.6 | 0.002 |
| II | Control | 161.7 | 0.03 |
| II | 2D | 58.7 | 0.01 |
| II | 3D control | ~0 (band only visible by eye) | ~ 0 (band only visible by eye) |
| II | 3D weight | 164.8 | 0.027 |
ELISA results
All of our collagen II sample absorbance values fell out of the low range of our assay and were barely measurable. For collagen I, only the 3D control supernatant, 3D control cells, and 2D cells had positive absorbance values after subtracting off background absorbance. Using a calibration curve derived from collagen I and II standards and subtracting background absorbance values off of all data, we calculated the following CN II: CN I ratios, which reflects the relative degree of chondrocyte-like phenotype. We believe these data give little valuable information because our collagen II levels were so low. The only conclusions we could draw are that the 3D control had were more chondrocyte-like than 3D weighted, and the 2D was even more chondrocyte-like than both 3D cultures. This contradicts our cell morphology and transcript analysis, where we found that the cells in 3D culture under mechanical stress exhibited the most chondrocyte-like phenotype while the 3D control and 2D cells were relatively de-differentiated into fibroblast-like cells.
| Sample | CN II: CN I supernatant | CN II: CN I cell | CN II: CN I supernatant | CN II: CN I cell |
|---|---|---|---|---|
| 2D | 0.0104 | 0.0038 | 0.0050 | 0.0024 |
| 3D control | 0.0074 | 0.0075 | 0.0039 | 0.0041 |
| 3D weight | 0.0028 | 0.0037 | 0.0014 | 0.0018 |
Wednesday/Friday Yellow
Background
We wanted to test how the strength of the alginate beads would affect cell growth and dedifferentiation. Thus, for our experiment we decided to use calcium and magnesium to form two different alginate bead scaffoldings. The concentration we used for magnesium was not correct and thus it did not form alginate beads. We then went ahead to test our calcium sample, but they were misplaced. Thus we ended up using another groups samples. They added a calcium chelating agent to one of there samples, which they thought would reduce the mechanical strength of the alginate scaffolding by stopping the G block interactions, which would cause more chondrocytes to de-differentiate into fibroblasts. There other samples acted as controls. Only 100,000 cells were initially seeded into the 2D sample and 1.5 million cells were seeded into the 3D samples. The chelating agent was added to 3D-sample 2 at a concentration of 1%.
Cell Recovery & Live/Dead Fluorescence Assay
Our cell counts were very low on day 3 for our 3D sample. We determined our 2D sample had around 900,000 cells/mL that were alive and around 11,000 cells/mL that were dead. On the other hand, our 3D sample only had around 30,000 cells/mL that were alive and around 11,000 cells/mL that were dead. The cells in our 3D sample had a round shape and were clustered together, while the cells in our 2D sample were elongated which matched a fibroblast morphology. Since we had low cell numbers, we took the maximum amount of each sample for our Live/Dead fluorescence assay.
RT-PCR & Agarose Gel
We started using another groups sample at this point and used there data for the RNA content. Below is the amount of RNA they used in their RT-PCR samples. We measured the collagen II to collagen I ratio of our samples by examining the RNA content as well as collagen expression of our samples. With the first half of our samples, we extracted all RNA for RT-PCR. The following is the amount of RNA we used in RT-PCR for each sample:
| Sample | RNA Concentration (ug/mL) | Max RNA (ng in 30 uL) |
|---|---|---|
| 2D | 104 | 200 |
| 3D Sample 2 | 18 | 200 |
We used 1/10th of the RNA concentration their group used in order to transcribe the collagen RNA to cDNA and then amplified this cDNA through PCR. Upon running our RT-PCR products on an agarose gel we only had bands for the 3D collagen 1 and 2D collagen 2, so we could not really compare the samples. Using ImageJ, to analyze the intensity of the bands and found the following ratios:
| Sample | CN-II : CN-I Mean Intensity |
|---|---|
| 2D collagen 2 | 40.459 |
| 3D collagen 1 | 68.550 |
Our bands were really close in size so we were able to determine the amount of DNA in each band relative to one another directly from the intensity. It appears that there is around 1.7X as much DNA in our 3D sample as our 2D sample. Which shows that if you just look at collagen types, it appears there is a high collagen I:II ratio. Since the samples came from 3D and 2D it is hard to compare them to conclude anything about chondrocyte phenotype development.
ELISA
We ran ELISA as a secondary method to measure the collagen I:II ratio. Our collagen one and our collagen two data was higher than the empty wells but below our blank. Thus, our sample data was out of range of the standard curve, so we couldn’t draw any real conclusions from the data. Although we couldn’t draw any conclusions, some absorbance was still seen. This means that there might of have been some collagen I and II present, but further experiments would need to be run.
Wednesday/Friday Green
We chose to vary the physical culture conditions. We originally planned to have four culture conditions: the 2D culture in a T25 flask, a 3D culture in 2% Sigma-brand low-viscosity alginate beads, a 3D culture in PEG-APGL-PEG-RGD and a 3D culture in a modified hollow-fiber bioreactor with 100 kDa cutoff cellulosic fibers. The PEG-RGD polymer contains the RGD peptide, which is an integrin binding sequence. We hoped that the cells would adhere to the polymer better with that peptide present. The bioreactor continuously circulates media and gas at a rate of 120 mL per minute and 27.5 mL per minute, respectively. It also puts shear stress on the cells at an adjustable quantity, which we hoped would stimulate the cells to produce collagen. Unfortunately, the PEG-APGL-PEG-RGD polymerization by UV light failed and made it impossible to work with our cells (e.g. exchange media). (Possible reasons for this were incorrect UV wavelength and old, degraded PEG and/or activator.) Also unfortunately, the bioreactor chambers melted during autoclave sterilization and were rendered unusable. This left us with just the first two described cultures (the 2D and 3D alginate). The 2D culture was seeded with approximately 500,000 cells, and the 3D culture was seeded with 1.1M cells (per replicate).
After seven days, we harvested some of our cells. We recovered 155,000 cells per mL from our 3D culture and 500,000 from our 2D culture. Then we did a Live/Dead© (Invitrogen) cell count assay. Images of our assay can be found on the Day 4(?) talk page. Our results were these:
| Culture | Recovery | Live | Dead |
|---|---|---|---|
| 2D | 500,000 cells/mL | 36 | 2 |
| 3D Alginate | 155,000 | 15 | 2 |
Based on this data, both 2D and 3D cultures were quite viable, though as expected the 2D culture was somewhat more viable. We also looked at our cells under 40X magnification with visible light to observe morphology. The 2D culture cells were mostly fibroblasts, where as the 3D culture cells were mostly chondrocytes. [Because the other groups all decided to add big, fancy tables, and we just decided to have one small table, we are compensating with a big, fancy border.]
We then did RT-PCR on the RNA from the samples to determine relative production of type I and II collagen. We loaded 200 ng of total RNA in our RT-PCR reactions. (Our total RNA concentrations were 570.4 μg/mL for 2D and 24 μg/mL for 3D.) The gel (shown in Day 5 Talk page, with lanes labeled there) showed a higher percentage of chondrocytes in the 3D culture than 2D culture, based on the relative amounts of mRNA present in the cells. (This is based on the fact that chondrocytes express collagen II in higher amounts than fibroblasts do.)
We did indirect ELISAs for both types of collagen. We normalized the amount of cells from the two cultures, using 1 mL of 3D culture and 400 μL 2D culture. Unfortunately many of our samples had concentrations below the range of our standards. We can say, though, that there was more collagen I produced than collagen II, according to these results. (Note that these results apparently contradict out transcript analysis results. This is more likely due to a poor regression equation.) We can also say that there was more collagen II produced in our 3D culture than in our 2D culture.
Wednesday/Friday Blue
Background and Set-up:
From our literary search we found that the rate of attachment of cells increases with cross-link density. So we planned on increasing the cross-link density of one our 3D cultures(from now on 3D experimental) to test if this increased the dedifferentiation of chondrocytes into fibroblasts. We accomplished this by increasing the calcium concentration ten-fold (1002 mM instead of 102 mM). Our 3D control sample used a standard calcium concentration (102 mM). We used a 2% alginate for both samples with G/M ratio of 70/30, because the G segments are involved in the cross-linking. We actually noticed a difference in the size of our alginate beads: The higher calcium concentration beads were much smaller in size. Our 2D sample was set-up at normal conditions. We plated the 3D cultures with about 1.3 million cells per well and the 2D culture with 100,000 cells.
Morphology and Cell counts:
We saw differences between our samples, as expected. The 2D culture cells looked more like fibroblasts than chondrocytes. They were very flat and spread out. The 3D control cells looked more like chondrocytes since they were large and spherical. The 3D experimental cells still looked like chondrocytes but they were significantly smaller in size than the 3D control cells. Our 2D cells had about 720,000 cells in total. Our 3D control and experimental samples had 49,500 cells total.
Live/Dead Cell Assay:
Unfortunately, since we had a low cell count and then we also had low cell recovery, we were not able to get good data from this assay. We saw about 4-5 live cells and 1-2 dead cells in the 2D sample. For our 3D control, we could more live than dead cells, but this was for a small sample size. The live cells were in a clump, so we were not able to count them.
Analysis:
When we prepared our cells for analysis, we had a low cell count for our 3D control sample and no cells in the 3D experimental.
Culture Type : Cell Count : Total RNA Concentrations
2D: 705,555 cells/ml: 144 ug/ml
3D control: 44,444 cells/ml: 20.4 ug/ml
3D experimental: 0 cells/ml (50,000 cells/ml dead): 5.2 ug/ml
Transcript-Level

Sample - CNII:CNI
2D - 2.067
3D control - 2.354
3D experimental - 0.120
The gel results are mostly consistent with our predictions. The 3D control and 2D samples show a high CN II:I ratio, indicating that a significant portion of the cells remained as chondrocytes. However, our 3D experimental sample shows a low CN II:I ratio. There are many reasons that account for the low ratio. It is possible that the chondrocytes de-differentiated into fibroblasts; but the low cell count in the 3D experimental sample does not allow us to make that conclusion.
Protein-Level

Our ELISA results are inconsistent with our transcript level results. This could be due a difference in the specificities of the two antibodies (cross-reactivity). Only the 3D Control pepsin digested had a measurable amount of collagen II, while all of the samples had collagen I. The 3D control pepsin digested Collagen II:I = 0.1685.
Wednesday/Friday Purple
Background/Experimental Setup
For our experiment, we decided to add a calcium chelating agent (mix of Sodium Citrate, EDTA, NaCl) to one of our 3D samples. We hypothesized that the addition of a calcium chelating agent would reduce the mechanical strength of our scaffold by inhibiting the G block interactions between our alginate beads. We predicted that this reduced mechanical support would be detrimental to chondrocyte phenotype preservation and would cause more chondrocyte de-differentiation to fibroblasts. The other 3D sample was used as a control (no calcium chelator), in order to have a basis of comparison for the level of de-differentiation. Our 2D sample was used to compare the effects of chondrocyte development in an environment lacking a scaffold. Due to low cell recovery, we initially seeded only about 100,000 cells in the 2D sample and about 1.5 million cells in each of our 3D samples. We added chelating agent to 3D-sample 2 at a concentration of 1%. We determined this concentration after some discussion of the fact that too high a concentration might completely obliterate our alginate matrix, while too low would yield no interesting results.
Cell Recovery & Live/Dead Fluorescence Assay
Upon trypan exclusion on day 3, we found cell counts to be extremely low. We determined that our 2D sample had around 500,000 cells/mL while each of our 3D samples had only around 11,000 cells/mL. Preliminary microscopy showed no difference in the morphology of our 3D samples. However, we noticed that most of the cells in our 2D sample had an oblong shape consistent with fibroblast morphology. Because of our low cell count, we took the maximum amount of each sample (1.5mL) for our Live/Dead fluorescence assay. Despite this, cell counts were too low for us to observe many live or dead cells.
RT-PCR & Agarose Gel
We measured the collagen II to collagen I ratio of our samples by examining the RNA content as well as collagen expression of our samples. With the first half of our samples, we extracted all RNA for RT-PCR. The following is the amount of RNA we used in RT-PCR for each sample:
| Sample | RNA Concentration (ug/mL) | Max RNA (ng in 30 uL) |
|---|---|---|
| 2D | 104 | 200 |
| 3D Sample 1 | N/A | N/A |
| 3D Sample 2 | 18 | 200 |
Although we did not detect any RNA in 3D-Sample 1, we suspected that there might be some RNA present. We decided to proceed with the max volume (20 uL) for RT-PCR of 3D-Sample 1.
Using collagen specific primers, we reverse transcribed the collagen RNA to cDNA and then amplified this cDNA through PCR. Upon running our RT-PCR products on an agarose gel we found that our Col II:Col I ratio was high in all of our samples. Using ImageJ, we analyzed the intensity of the agarose gel bands and found the following ratios:
| Sample | CN-II : CN-I Mean Intensity |
|---|---|
| 2D | 25.6 |
| 3D Sample 1 | 18.0 |
| 3D Sample 2 | 13.8 |
Although our transcript-level analysis shows the Col II:I ratio of 3D-Sample I to be higher than that of 3D-Sample II, the difference is not significant enough to conclude whether chondrocyte phenotype development was hindered by the calcium chelator. Furthermore, since we are unsure of the initial amount of RNA present in 3D-Sample 1, it is difficult to compare the ratios accurately.
ELISA
We performed ELISA in order to analyze the Col II : Col I ratio of our samples. Following is the initial volume of cells in 1 ml which we started with.
| Sample | # Cells |
|---|---|
| 2D | 922,222 cells |
| 3D Sample 1 | 33,333 cells |
| 3D Sample 2 | 44,444 |
The results of our ELISA were overall unreliable and generally incomparable to our transcript-level analysis. We noted a few mistakes in protocol which reduced the validity of our data. First, we added primary antibody to our standards, but forgot to add the primary antibody to our samples the day we were supposed to. Thus, our standards sat with antibody and then block buffer, however our samples were sitting in dry wells overnight. We attempted to correct this mistake by adding primary antibody the next day, but knew our results were going to be questionable. Second, by the time we were ready to add secondary antibody to our plates, block buffer was out of stock. As such we diluted our secondary antibody in PBS rather than block buffer. Also, because we developed our plates 1 day after other groups, the development buffer had been left out at room temperature for > 24hrs. These mistakes in protocol coupled with our low initial sample recovery contributed to the poor results of our ELISA.
Though we did not expect to obtain significant results, we nevertheless ran our ELISA. Our Collagen I sample was left to develop overnight. As such, we observed a small amount of absorption in these wells. Upon comparison to the standard Collagen I curve, we found this data to be unreliable since the highest OD value was still lower than the lowest value on our standard curve. In other words, our sample data was out of range of our standard curve. Though we cannot extract concrete information about the Collagen I content of our samples from this data, the fact that there was any absorbance at all suggests that Collagen I might be present. We would however need to redo the experiment (possibly modified) to conclusively state this.
Our Collagen II samples showed no absorbance at 420 nm. Perhaps if these samples had been allowed to develop overnight some activity would have been observed. Since we did not obtain any Collagen II data from ELISA, we cannot make any comment on the ratio of Col II: Col I based on this assay.
Wednesday/Friday Pink
Our Experiment
For our experiments, we chose to test the effects of mechanical compressive stress on chondrocyte phenotype. Our 2D sample was cultured normally. Both of our 3D samples were cultured in Petri dishes: the control had no weight added and was cultured in 1% alginate, our test sample was compressed using the bottom of the inverted Petri dish, covered with filter paper to allow diffusion of nutrients and oxygen into the beads. We hypothesized that in compressive situations, similar to those seen by the cartilage in the knee, would result in maintained chondrocye phenotype.
Cell Observations
Observation of the cells after one week of growth confirmed our prediction, at least initially. Our 2D sample was mostly spread cells, resembling fibroblast morphology more so than chondrocyte morphology. It was hard to tell what happened with our 3D samples, but the cells within the beads looked circular. We recovered 356000 cells/mL of our 2D sample, and less of both of our 3D samples; 33300 cells/mL for the weighted sample and 116600 cells/mL for the control sample.
Live/Dead assay
Our Live/Dead assay revealed very few dead cells, but also very few lives cells. This is most likely the result of relatively low recovery and incomplete staining. We think, after looking at the cells under normal lighting that it was a staining problem, and that most of our cells remained alive regardless of conditions.
Cell Concentrations
Concentration analysis of our samples showed high RNA amount in two out of three cell conditions. Our 2D sample had a concentration of 120.4 ug/mL, 3D control was 32.4 ug/mL, and 3D weighted was very low, at 6.8 ug/mL. We decided to run the full 200 ng of RNA in each RT-PCR reaction, but ran out of our 3D weighted sample. We ended up using only 75% as much for the 3D weighted as for the other two.
RT-PCR Results
We performed RT-PCR on all three of our samples, but got very little yield. Our 2D samples yielded a strong band of collagen II (around 400bp), and a very faint but visible on the gel (not on the picture) around 100bp that was collagen I. None of our 3D samples showed visible bands, despite the high RNA concentration evidenced in spectrophotometer. Regardless, based off the striking results of our 2D sample, we can conclude that even the cells displaying the more fibroblast phenotype were still producing markedly more collagen II than collagen I. Using the 2D results on the agarose gel, our collagen II to collagen I ratio was 23.25 (100/4.3).
| Lane | Content | Volume | Observation |
|---|---|---|---|
| 1 | Ladder | 11 uL | weak |
| 2 | 2D Collagen I 5uL | 18 uL | very weak |
| 3 | 3D Control Collagen I 5uL | 18 uL | not visible |
| 4 | 3D Weighted Collagen I 5uL | 18 uL | not visible |
| 5 | 2D Collagen II 5uL | 18 uL | strong band |
| 6 | 3D Control Collagen II 5uL | 18 uL | not visible |
| 7 | 3D Weighted Collagen II 5uL | 18 uL | not visible |
| 8 | Collagen I Mock | 18 uL | no band |
| 9 | Collagen II Mock | 18 uL | no band |
ELISA Result
Our ELISA results were inconclusive, because our Collagen II data was not within the bounds of the assay. The Collagen I assay worked, however, only when it was oversaturated. Interestingly, our pepsin cell samples had less protein yield than our non-pepsin cell samples. We also had very high background.
(sup = supernatant, c = control, w = weighted) Collagen I (y = 0.0456x + 0.0065) Collagen II (y = 0.0519x + 0.2045)
Our Collagen Ratio Table:
| Sample | Collagen I (in ug/mL) | Collagen II (in ug/mL) | CI : CII ratio |
|---|---|---|---|
| 2D Sup | 2.65 | -3.55 | NA |
| 2D cell | 10.34 | -3.51 | NA |
| 3D C Sup | 4.72 | -3.66 | NA |
| 3D C Cells | 2.08 | -3.51 | NA |
| 3D W Sup | 1.23 | -3.33 | NA |
| 3D W Cells | 0.86 | -3.66 | NA |
Within Collagen I our results make sense: Our weighted sample (under compressive stress) shows the least amount of Collagen I, thus we can assume it had higher levels of Collagen II and kept its chondrocyte phenotype.
Our data was normalized to 3D control, using 187000 cells (for both 3D control and 2D). The weighted 3D sample did not have enough, and thus we used half of what was left (from setting aside a certain amount for RT-PCR), for a total of 16700 cells. As such, our weighed sample was not normalized to the other two samples used.