Todd:Catalytic, Asymmetric Pictet-Spengler Reaction
The Catalytic, Asymmetric Pictet-Spengler Reaction
Katrina A. Badiola, School of Chemistry, The University of Sydney, NSW 2006, Australia
Murray N. Robertson, School of Chemistry, The University of Sydney, NSW 2006, Australia
Michael A. Tarselli, Biomedisyn Corp., Woodbridge, CT, United States of America
Matthew H. Todd, School of Chemistry, The University of Sydney, NSW 2006, Australia
Additional authors - add alphabetically if you contribute something substantial (e.g., the summary of a paper with a scheme). Please include some public place you can be contacted, e.g. a G+ account.
The licence for this page is CC BY 3.0
How this works:
0. Background described in this blog post.
1. This article will be a stand-alone review. It will be written openly in January and February 2012. Once it is complete and well-written, it will be submitted to an open access journal for publication. This will provide a citation - a static object to which others may refer. Until that time this page is a work in progress and is not to be taken as complete.
2. The article is open source, like Wikipedia. Anyone can add and edit. To contribute you will need to get an account on OWW (quick and easy). People contributing something lasting and substantial will be named authors. Final arbitration on what qualifies as authorship lies with Mat Todd, who is the corresponding author for this paper.
3. References for the papers described here may be found in full at the Mendeley page. Chemdraw files for the schemes are currently held in a Dropbox folder we can share with anyone who needs it. PNG files are hosted on this wiki site.
4. The review incidentally acts as background to the open science project to find a catalytic, asymmetric route to praziquantel. That project is currently active in the lab. A catalytic, enantioselective synthesis of this drug is an alternative to the resolution approach that was discovered, also by open science.
5. If you want to get in touch to ask questions (e.g. if you want to ask "what can I do to help?") please do not use email. The current to-do list is on the talk page (click on the tab above). Questions should go there with your initials. You can also discuss via Google+ pages: Mat, Kat, (please add other public places where you can be contacted if you contribute as an author by inserting links along with your name, above).
6. When editing remember to include a reason for your edit, to make it easier to revert back to an original version if needed.
Important note on simultaneous edits: If you are intending to work for some time on editing the page, we'd recommend writing text elsewhere then pasting it in here, since there is a small but non-zero chance that you might simultaneously edit the same section as someone else, resulting in the chance of the loss of some information.
Schemes: Use Wiley/Angewandte settings for the .cdx files and add below as 300 dpi .png files.
References: Please use the citation style below (click to see the page source for formatting details) and include a link to the DOI of the paper to that it may be checked easily.
Introduction
The Pictet-Spengler (PS) reaction is just over a century old.Pictet, Spengler 1911 The reaction is a cyclization between an amine that carries an aromatic ring, and an aldehyde, usually catalyzed by acid (Scheme 1911 Pictet Spengler 2). The original reaction employed formaldehyde and phenethylamine, to give the tetrahydroisoquinoline scaffold. Several years after the original report tryptamine was found to be perform well in the reaction permitting access to a range of tetrahydrocarbolines.[ref] A wide range of variations on these original themes have been investigated.Stöckigt 2011, Whaley, Govindachari[Youn 2006][Larghi 2011] such as cases where the amine component is acylated or alkylated, those where ketones are employed rather than aldehydes so as to generate quaternary centres adjacent to the aromatic ring, and reactions employing alcohols rather than amines - the so-called oxo-PS reaction.

The reaction is important for two reasons. Firstly, Nature uses this chemistry. Enzymes ("pictet spenglerases") carry out the PS cyclization to produce important intermediates which feed into many biological pathways that result in bioactive small molecules, such as strychnine, morphine, vinblastine and reserpine.

All monoterpene indole alkaloids are thought to be made via this route, the key intermediate of which is strictosidine, formed from a PS reaction between tryptamine and secologanin.

Secondly, and probably consequently, the general structures one can access through the PS reaction - alkaloids with a stereocentre adjacent to an aromatic ring - are often highly bioactive, and are of interest for the development of new medicines. This has led to a great deal of interest in controlling the stereochemical outcome of PS reactions. Most of this work has involved understanding diastereoselective PS reactions, where an existing stereocentre in the molecule, often derived from an amino acid, directs the ring closure.Cook 1995(Need: What was the first diastereoselective example? Cook 1992 Cook[Larghi 2005, review, no DOI][Youn 2006] This approach has been used in several notable total syntheses, among them (-)-suaveoline (Cook, 1992) and (-)-phalarine (Danishefsky, 2010). (Need: combine the two structures below with the general scheme for this part)

Several important pharmaceuticals may be synthesized with the PS reaction, for example the widely-used anthelmintic praziquantel, tadalafil (Cialis(TM)) used for erectile disfunction, the painkiller Etodolac and the promising new antimalarial compound NITD609 (Scheme Intro - Relevant Drugs). The biological activity of these compounds typically arises from one enantiomer: for example the (S) enantiomer of Etodolac (10.1021/jm00366a025) and the (R)- enantiomer of praziquantel (10.1371/journal.pntd.0000357); ent-NITD609 is inactive (10.1126/science.1193225). The potency of this class of compounds leads to great market value: $1.7 Bn for tadalafil alone in 2010.(Need: full reference inserting at end)(Eli Lilly Annual Report 2010, available at http://investor.lilly.com/annuals.cfm) Efficient enantioselective methods for the PS reaction would be very desirable, and progress to date in this field is the subject of this review. It is now certainly possible to carry out some PS reactions to give products with high enantiomeric excess. However, the scope of these processes is limited (as we shall see) reducing their impact on the preparation of bioactive compounds industrially. The PS reaction has been used in the racemic industrial synthesis of X, for example, but the enantiopure material is obtained through a resolution.(Need: example here - someone please check http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3107522/)

Enantioselective approaches, where the stereocontrol of the cyclization is not governed by a stereocentre already in the cyclization precursor, have received much less attention than the diastereoselective, racemic or achiral version of the PS reaction. It was shown in 1996 that the PS reaction could be effected with superstoichiometric Lewis acid - in this case the cyclization of the (Z)-nitrone derived from Nb-hydroxytryptamine using diisopinocampheylchloroborane (Ipc2BCl) to give the corresponding tetrahydro-β-carboline products (Scheme Nakagawa 1998).(10.1016/0957-4166(96)00134-6 and 10.1021/jo980810h) The racemic cyclization could be effected with Bronsted acid and a number of achiral Lewis acids, but it was found that Ipc2BCl gave high yields and ee with reduced temperatures. Lowering the quantity of Ipc2BCl to 0.5 eq. caused a significant reduction of yield, while attempts to alter the Lewis acidity by substitution of the chloride (with e.g. fluoride or triflate) did not improve yield or ee. The proposed reaction mechanism involved the formation of an iminium ion with coordination of the nitrone oxygen to the Ipc2BCl boron centre, and preliminary modeling confirmed a difference in transition state energies for approach of the indole from the re- and si-faces of the iminium ion. It was found that iminium ions derived from electron-deficient aldehydes gave poor enantioselectivity, but that these cyclizations were effective (with 74% ee in the case of the nitrone derived from 4-nitrobenzaldehyde) when a different reagent was added, a boronate incorporating two BINOL ligands (X) that had been previously described by Yamamoto for aldol-type reactions of imines.(10.1021/ja00102a019) The mechanism of action of such a boronate was suggested to involve replacement of one of the coordinating BINOL oxygen atoms with that on the nitrone, and it is not clear why such a mechanism could not operate catalytically. Nevertheless this was the first report of a reagent-controlled enantioselective PS reaction; earlier work by Nakagawa employing a similar approach had led to cyclization, but to give enantioenriched spiroindoline products.Paper Chiral Bronsted acids were ultimately successfully applied to the catalytic, enantioselective PS reaction through the use of a phosphoric acid, rather than a boronate, as described below.

A chiral Lewis acidic silane reagent (X, Scheme Leighton 2009) was shown to be effective in promoting highly enantioselective PS reactions.(Leighton 2009 10.1002/anie.200806110) The substrates (e.g., X) contained α-ketoamide ketimines: NMR studies suggested that following O-silylation the proton from the NH group transfers to the reagent's nitrogen atom and activates the complex (X) to cyclization. Electron-withdrawing groups on the N-aryl ring improved the reaction rate. The quaternary stereocentre could be generated even with sterically demanding aryl groups appended to the imine, such as 1-naphthyl (product ee 87%). A one-pot procedure was also developed in which the initial amine and a-ketoamide were allowed to react, followed by the addition of the reagent. The process was shown to be effective on a 5 mmol scale with 1.3 equivalents of silane, and the pseudoephedrine ligand could be recovered and crystallised following work-up of the reaction. It was subsequently shown that the same (now commercially-available[Leighton 2010]) reagent could be used with similar effectiveness in the synthesis of the more ususual PS reaction products X and X, derived from the less common indole amines (1H-indol-4-yl)methanamine and 2-(1H-indol-1-yl)ethanamine.[Leighton 2012 10.1021/ol300922b] The latter heterocyclic framework had just been synthesized using the enzyme strictosidine synthase (see section X).

It is perhaps surprising that there are still no examples of Lewis acid-catalyzed asymmetric PS reactions. In a recent study (10.1016/j.tetlet.2011.08.071) a small number of chiral Lewis acidic complexes were shown to effect conversion in a PS reaction between tryptamine and isatins, but there was negligible enantioinduction; high product ee was ultimately achieved with Bronsted acid catalysis, to which we now turn.
Brønsted Acid Organocatalysis
(Note - consider a diagram section at start which includes the structures of all the BINOL-derived catalysts so we don't have to include in the individual schemes). i.e. we draw them at start and then number them then just include the numbers in the schemes.
Chiral Brønsted acids have been shown to be effective in the catalytic, asymmetric PS reaction, which builds on earlier work demonstrating the ability of such compounds to catalyze the reaction between nucleophiles and iminium ions.
Akiyama (10.1002/anie.200353240) reported chiral phosphoric acids prepared from arylated BINOLs in the enantioselective Mannich-type coupling of silyl enolates with aldimines (Scheme Akiyama 2004). High yields and enantio- (as well as, in appropriate cases, diastereo-) selectivities were observed with a variety of substituted aldimines and enolates (What's "high" for this reaction? Akiyama reports 81-96% ee, and given that the group reports several "100% yields" I'm inclined towards suspicion -MAT). Limitations to the methodology were that an ortho hydroxy group was required on the N-aryl ring of the aldimine (Why? Mechanistic raionale? - MAT), and that aldimines derived from aliphatic aldehydes did not participate effectively. Typical catalyst loading was 10 mol%. The structure of the catalyst itself may be thought of as a chiral proton, i.e., a proton surrounded by a chiral environment, particularly given the aromatic rings of the BINOL and the 3-substituents are non-coplanar. However, the proposed mechanism operates via an ion pair of phosphate and iminium ion. The bond-forming event would naturally disrupt such an ion pair, ensuring catalytic turnover. (ultimately need a scheme of this very general idea [nice example in the Akiyama paper here] but may go in mechanism section)

At the same time Terada (10.1021/ja0491533) reported similar catalysts in the enantioselective Mannich reaction for the synthesis of β-aminoketones (Scheme Terada 2004a), utilizing acetylacetonate nucleophiles in place of silyl enolates. Terada, like Akiyama, notes the important influence of the 3,3'-position of the naphthyl rings on the enantioselectivity of the reaction. A followup paper later that same year incorporates furan nucleophiles (10.1021/ja046185h).

Since these early examples, several reactions have been reported that can be catalysed by these or related structures (reference reviews of chiral bronsted acid catalysts here).(Terada 2010 Bull Chem Soc Jpn 10.1246/bcsj.20090268)
List reported the first Brønsted acid-catalyzed enantioselective Pictet-Spengler reaction in 2006 (10.1021/ja057444l). Chiral, substituted phosphoric acids were shown to be effective in the PS cyclization of tryptamines with a number of aliphatic and aromatic aldehydes (Scheme List 2006). The diester functionality was found to be necessary, presumably due to promotion of a clean reaction through the Thorpe-Ingold effect (and an aldol side reaction was observed when the esters were absent). Lower yields were typically observed when the methoxy group was absent from the tryptamine aromatic ring.

In 2007, Hiemstra reported the enantioselective synthesis of tetra-β-carbolines via the in situ formation of N-sulfenyliminium ions (10.1002/anie.200701808). Stabilization of the intermediate iminium by the N-tritylsulfenyl group was effective at promoting the acid-catalyzed PS reaction by substituted enantiopure binaphthyl-derived phosphoric acids. Several substitutions were assayed in the 2-position of the catalyst, with no clear trend being observed in the ee of product obtained. The N-S bond in the N-tritylsulfenyl product was found to be susceptible to homolytic cleavage, but this could be suppressed by the addition of a radical scavenger. A one-pot process was developed that allowed precipitation of the product as a salt, and this was applied to the synthesis of a variety of substituted tetra-β-carbolines with high yield and high ee. The reaction was also demonstrated on a multi-gram scale.

An extension to this methodology was developed that allowed the synthesis of enantioenriched N-benzyl-protected versions of similar products from the relevant protected tryptamines and diverse aldehydes (10.1021/jo8010478). During optimization it was found that removal of water was essential for high enantioinduction presumably because water prevents effective association between catalyst and cyclization precursor. Several control reactions were performed under the optimized conditions that suggested this PS reaction was irreversible. The best-performing catalyst was the triphenylsilyl-substituted binaphthyl system, delivering up to 100% conversion and high ee values (78-85%). The ee obtained was sensitive to the aldehyde employed. Of the aliphatic aldehydes, no product was observed with the enolizable phenylacetaldehyde and low ee (8%) was obtained with 3-phenylpropanal. While electron-deficient aromatic aldehydes generally gave products with high ee as expected, there were exceptions that performed poorly; 3-chlorobenzaldehyde gave near-racemic product, for example.
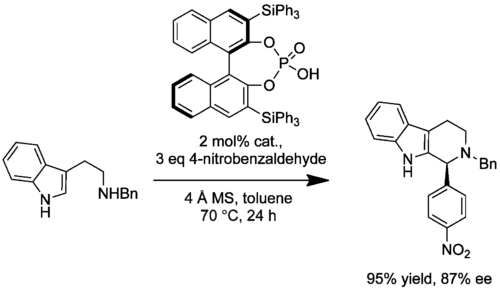
This methodology has been employed in the syntheses of three natural products. The PS reaction employed in the synthesis of (-)-arboricine (10.1021/ol900888e) (Scheme Hiemstra 2009) involved an aldehyde containing a dioxolane-protected ketone group, preventing an aminal formation that was observed when the ketone was used unprotected, but it is notable that this protecting group withstands the PS cyclization, and that the yield and ee of the cyclization were both dramatically improved by the use of the protecting group. The partially saturated (and slightly more sterically crowded) (R)-H8-Binol-PA catalyst was also shown to be effective. This catalyst was subsequently used for the key step in the synthesis of (+)-yohimbine (Scheme Hiemstra 2011).(10.1021/jo201657n). The natural product was to be synthesized via a Diels-Alder precursor that could itself be made using an enantioselective PS reaction. However, the aldehyde required for the PS reaction was β,γ-unsaturated and this was likely to result in the unproductive formation of an enamine from the initially-formed iminium ion. This substrate limitation necessitated use of a latent double bond, in this case a phenylselenide; this group survived the successful PS cyclization and could be eliminated to the double bond via oxidation to the selenoxide. A similar synthetic strategy was employed in the synthesis of the related corynanthe alkaloid family. (10.1002/chem.201103150)


Dixon included a chiral phosphoric acid as part of a reagent cocktail effecting a cascade sequence involving a Pictet-Spengler-like cyclization (Scheme Dixon 2009).(10.1021/ja9024885) Tryptamines and lactones formed ketoamides with an appended π-nucleophile that underwent enantioselective cyclizations in the presence of chiral phosphoric acids, and it was again shown that aromatic substitution of the BINOL ring system was essential for high ee. The method could be used with more substituted lactones to effect high levels of diastereocontrol: the combination of a disubstituted enol lactone with tryptamine gave isolable intermediates, the structures of which implied that the formation of the reactive iminium ion was fast and reversible, and that final ring closure occurred with one matched catalyst/substrate pair. The mechanism of the enantiodetermining cyclization is presumed to be via a tight ion pairing between iminium ion and catalyst conjugate anion. High yields and stereoselectivities could be obtained for diverse products using this methodology, which was shown to be compatible with a one-pot cascade process that also included a gold(I)-catalyzed step to generate the initial lactone.

The method was broadened to allow the use of racemic keto acids and esters in place of the enol lactones, again with polycyclic products being produced in high yield and ee (79-98%). (10.1021/ol101651t). If the reaction time was reduced, enamides could again be isolated, one achiral and the other chiral with an ee of only 7%. Either enamide gave the intended product with an ee of 83% when resubjected to the reaction conditions, supporting the fast, reversible formation of iminium ions which are trapped by an enantioselective cyclization event controlled by the chiral acid. Possibly mention the racemic oxo acid.

Franz (10.1016/j.tetlet.2011.08.071) screened a number of catalysts in the search for a means of creating medicinally-relevant spirocyclic structures from tryptamine and isatin. Lewis acidic complexes were ineffective, and though thioureas gave some enantioinduction, it was found that Bronsted acid catalysts were the most effective, giving products in sometimes excellent ee in often near-quantitative yields. Interestingly the 3,3'-substituents on the BINOL ring system strongly influenced the enantioinduction, to the extent that changing this substituent (from e.g., anthracenyl to triisopropylphenyl) reversed the sense of enantioinduction (strictly, the (S)-enantiomer of one catalyst gave the same enantiomer of product as did the (R)-enantiomer of the other catalyst.) Unsurprisingly the outcome of the reaction was dependent on the choice of solvent as well as the electronic and steric substitution pattern of both coupling partners. (Citations done, but leads to 10.1002/adsc.201100050 - MHT doing Jan 23 - very similar, which may require a proper merge here)


Thiourea Organocatalysts
In 2004, Jacobsen reported his initial work on asymmetric catalysis of the acyl-Pictet-Spengler reaction using chiral thioureas. Jacobsen realised the inherent challenge of developing an asymmetric Pictet-Spengler catalyst involved low reactivity of the imine substrate. Additionally, previously reported racemic efforts had involved Lewis acid catalysts paired with highly reactive agents at high temperatures. Jacobsen enhanced the reaction by increasing the electrophilicity of the iminium intermediate through formation of the corresponding N-acyliminium ion (MAT - Why? We need to explore this concept a bit...) Early screening experiments showed cyclization occuring at -30 °C in 59% ee. While screening individual reaction parameters, Jacobsen discovered that product chirality exhibited a strong dependence upon the structure of the acylating agent, reaction solvent, and temperature.

Under optimized conditions (-30 °C, ether, 5 mol% catalyst), Jacobsen's thiourea delivered enriched tetrahydro-B-carbolines in 65-81% yields, and up to 95% ee.
A proposed mechanism for the thiourea-catalysed enantioselective Pictet-Spengler-Type cyclization was published by Jacobsen in 2007. Interestingly, key experimental observations, supported by DFT computational analyses, pointed towards an SN1-type pathway in these cyclizations, with catalysis via a previously unprecedented anion-catalyst hydrogen bonding mechanism.
An extensive screen of acidic additives revealed that either chlorotrimethylsilane or the combination of HCl and 3 Å molecular sieves afforded high levels of conversion and enantioselectivity, but that water had a deleterious effect on catalyst activity. Furthermore, a quite significant inverse correlation between conversion and reaction concentration was observed, with reactions run at lower concentrations affording substantially improved yields.
As a direct demonstration of the applicability of this new methodology, Jacobsen applied the enantioselective hydroxylactam cyclization to the total synthesis of (+)-harmicine with the cyclization proceeding in 97% ee followed by subsequent LiAlH4 reduction affording the natural product in only four steps from tryptamine. (Note - picture reads "Hermicine" - MAT)

Variable temperature 1H NMR studies of reaction mixtures indicated that formal dehydration and formation of the corresponding chlorolactam is rapid and irreversible. Further observation of enhanced reactivity of alkylated versus reduced derivatives suggests that an SN2-type displacement of chloride is not operative in the cyclization reaction, which points instead to an SN1-type mechanism. Since the enantio-determining step is likely either the addition of the indole to the N-acyliminium ion (b → c or b → d), or alkyl migration of the spiroindoline intermediate (c → d), catalyst interaction with at least one of these species is required. However, there is no viable Lewis basic site for catalyst binding to substrate in c or d.

Therefore, Jacobsen proposed that the thiourea catalyst promotes enantioselective cyclization by inducing dissociation of the chloride counterion and forming a chiral N-acyliminium chloride-thiourea complex. Noticeable halide counterion effects and solvent effects on enantioselectivity lend proof to this theory. Furthermore, it was suggested that catalysis and enantioinduction may result from initial abstraction of a chloride anion from a in an SN1-type rate determining step (a → b) and subsequent cyclization mediated by the resulting anion-bound thiourea. This mode of catalytic generation of cationic intermediates was previously reported in the well-established anion-binding properties of ureas and thioureas. Further, the possibility of high levels of enantioinduction induced through counterion interactions is well precedented in chiral phase-transfer catalysis and has recently been demonstrated in the context of asymmetric counterion-directed catalysis.
In 2007, Jacobsen published a review titled “Small-Molecule H-Bond Donors in Asymmetric Catalysis” identifying chiral hydrogen-bond donors used for enantioselective synthesis. The area regarding to the PS reaction referred to previous work reported by Jacobsen. Concluding, Jacobsen stated his surprise at both phosphoric acids and thiourea derivatives being capable of mediating enantioselective transformations of prochiral iminium and N-acyliminium ion intermediates as they exist in opposite ends of the spectrum of the pKa scale of known H-bond donor catalysts.
In 2008, Jacobsen put his previously discovered enantioselective thiourea-acyl-Pictet-Spengler catalyst to use in the total synthesis of (+)-yohimbine. The synthesis was achieved in 11 steps and 14% overall yield with the absolute configuration of the molecule being established through the highly enantioselective thiourea-catalyzed acyl-Pictet-Spengler reaction at the start of the synthesis.

In 2009 Jacobsen reported asymmetric Pictet-Spengler reactions cocatalyzed by a chiral thiourea and benzoic acid. A number of optically active tetrahydro-β-carbolines were obtained in high ee.

The catalytic cycle for this was proposed where imine protonation is induced by a thiourea catalyst via H-bonding to the conjugate base of a weak Bronsted acid additive. The highly reactive protioiminium ion then cyclizes and aromatizes to generate the desired product and Bronsted acid cocatalyst. Examples also show that this thiourea catalyst promotes highly enantioselective Pictet-Spengler reactions on electronically and structurally diverse substrates.

In 2011, Jacobsen, Lee, and Klausen published further work on a thiourea/benzoic acid cocatalyzed "iso-Pictet-Spengler" reactions, so named due to the alternate connectivity of the 2-substituted isotryptamine starting material. This altered substrate permits synthesis of optically pure tetrahydro-γ-carbolines. Jacobsen reports a straightforward procedure for upgrading the produced tetrahydro-γ-carbolines' enantiopurity through Boc protection of the free amine, followed by crystallization or trituration. This simple preparative step elevates the ee to >99% in nearly all published examples. The group's optimized conditions are applied to a single aromatic ketone, generating a quaternary center with high ee (98% post-trituration), albeit with reduced yield (53%, 2 steps). Jacobsen cautions that one limitation of the method is the need for a slight excess of the thiourea catalyst relative to BzOH, to avoid a deleterious racemic background reaction.

What is Known of the Mechanisms of Existing Systems
Terada (10.1021/ja0491533) mentions (wrt Bronsted acids) "1) Tetradentate structure around the phosphorus(V) atom would prevent free rotation at R of the phosphorus center by formation of a ring structure. This characteristic feature cannot be found in other possible Brønsted acids, such as carboxylic and sulfonic acids, etc. 2) Their appropriate acidity16 should catch up the imine through hydrogen bonding without loose ion-pair formation. 3) Their phosphoryl oxygen should function as a Lewis basic site, and thus a phosphoric acid could function as a bifunctional catalyst."
Interesting --> Cook et al, "Study of the Cis to Trans Isomerization of 1-Phenyl-2,3-disubstituted Tetrahydro-β-carbolines at C(1). Evidence for the Carbocation-Mediated Mechanism" Paper - Proposes mechanism for the racemisation via retro Pictet-Spengler of enantioenriched tetrahydro-β-carbolines synthesised from tryptamines and aldehydes.
For binaphthyl-derived phosphoric acids are there any trends in the nature of the substituents vs. ee obtained? In Hiemstra 2007 no clear trend is visible in Table 1. Franz noticed strong effect of 3,3'-substituents, with similar sterically-demanding groups reversing enantioinduction.
Limitation: avoiding β,γ-unsaturated aldehydes, which tend to tautomerise from the intermediate iminium ion to the unreactive, conjugated enamine, e.g. in Hiemstra 2011.
Dixon 2010 (10.1021/ol101651t). Both isolated enamide intermediates (epimers?) gave the same ee on treatment with the chiral BINAP (to drive rxn to completion). The proposed mechanism was that both reactions underwent rapid epimerisation through a common prochiral enamide intermediate (steady state?). Also, suggested enantioselectivity arose from facial differentiation imposed by the tight ion pair between the binol phosphoric acid conjugate base and the iminium ion.
Terada Review (10.1246/bcsj.20090268): Phosphoric acids as stronger Bronsted acids than thioureas or than TADDOL (used in the Rawal Nature paper). Considered other possible acids including sulfonic (too strong), carboxylic and sulfuric (free rotation problem), and phosphoric - just right, and chiral info is closer to proton. (When deprotonated, the O minus and P=O sites interconvert, right, but this is unimportant?) Phosphoric acids not expected to form loose ion pairs. Expected to be H-bonding etc that keeps components together. Ring system employed in the BINOL derivatives makes more rigid. Good? Mechanistic proposal in Figure 4. H-bonding network, not ion pair. Developed phosphorodiamidic acid in Synlett 2006, 133. Figure 11 has mechanistic cycle that may be of interest to PZQ. Do all the enecarbamate reactions known to function have N-H's?
Solvents: toluene found to be a good solvent for a number of these reactions, e.g. 10.1021/ja9024885. No clear trend observed in Franz 2011; DCM happens to be the best, but...
Thiourea mechanism: Franz 2011 has Jacobsen ligands as giving good conversion but moderate ee, but the Takemoto ligand giving no conversion.
Clearly the main issue with regards the mechanism is the need for an electron-rich ring for the PS reaction to occur. Franz 2011 looked at this a little, though there are two steps in the mechanism - imine formation and cyclization, so one needs to be careful interpreting results.
Strictosidine Synthase Mechanism: O'Connor 2008 compares acid-base effects of non-enzymatic aqueous solution vs. enzyme-catalysed reactions (using kinetic isotope effects). The rate-determining step appears to be the same for both systems. O'Connor also discusses the pH dependence of enzymatic catalysis, but not for binding of substrate, and proposes an enzyme mechanism involving key residue Glu309; deprotonation of tryptamine increases its nucleophilicity for aldehyde attack (emphasis on proximity of the Glu residue to the substrate). Lack of significant conformational change on binding of the substrate.
O'Connor 2010 TL - A computational model was generated for the OpSTS active site, which determined that a reversible mixture of diastereomeric intermediate carbolines were formed, but that only the 2(R)3(S) diastereomer was capable of subsequent deprotonation by key Glu309, a carboxylate residue in the active site.
Enzymatic Catalysis
(Aim of this section should be to describe preparative uses of these enzymes, and whether they are able to do reactions we can't with small molecules. Mechanistic insights (crucial here) should go in the mechanism section, to provide a comparison with what's known of small molecule systems.)
Stockigt and Waldmann's 2011 ACIEE review on the Pictet-Spengler reaction eloquently opens with discussion of the two known families of "Pictet-Spenglerases" - enzymes that take as their substrates an electron-rich aromatic, appended to an ethylamine, and an aldehyde - and transform them into asymmetric tetrahydroisoquinoline or tetrahydro-β-carboline motifs. Strictosidine synthase (STR1), first isolated in 1975 by Scott and Lee, cyclizes natural product precursors belonging to the strychnos and ajmaline alkaloid pathways. Norcoclaurine synthase (NCR), isolated in 1981 by Nagakura from plant cell cultures, which condenses tyrosine-derived aldehydes and dopamine to form a variety of benzoisoquinoline precursors.
A variety of different halogenated and alkylated tryptamine precursors have been incorporated into final alkaloid structures utilizing a multi-enzyme cascade. First, the group transferred the genes encoding tryptamine halogenase RebH (from soil bacteria) into C. roseus hairy root culture. They then developed a modified STR enzyme, STRvm, capable of recognizing and turning over halogenated Trp precursors. The group used MS and 2D NMR methods to confirm detection of downstream halogenated alkaloids. (O'Connor, Nature 2010, 461)

In 2010, O'Connor and coworkers reported 3 strictosidine synthase homologs, isolated from R. serpentina (RsSTS), C. roseus (CrSTS), and O. pumila (OpSTS). Previous research by O'Connor and Stockigt had shown that variations to the tryptamine synthon (electron-rich, electron-deficient) were tolerated by the enzymes, but that aldehydes other than secologanin were not turned over. The O. pumila isolate, a lower homolog (~60% sequence identity to RsSTS or CrSTS) was capable of catalyzing the PS reaction between tryptamine and various non-native aldehydes. Tetrahydro-B-carbolines thus formed had >98% ee.

The substrate tolerance of strictosidine synthase was further extended by Stockigt and a multi-institutional team in a JACS 2012 paper. Strictosidine synthase (STR1), after substrate-directed mutagenesis, was able to turn over heteroatom-containing tryptamines such as 7-N tryptamine (indazole ethanamine). The resulting secologanin conjugate was detected by HRMS.
Miscellaneous Related Systems, or Known Catalysts not yet used for the PS
This is a very important section where we describe some obvious things that can be tried next in the field. Reviews are not proposals, but making maps gives you a clear sense of what has not yet been explored.
Non-BINOL derived Bronsted acids (other relevant, perhaps tartrate-derived, biologically relevant?)
Internal Lewis-acid assisted urea catalysis (see: Mattson, OL 2011 and 2012)
Enzyme mutagenesis to accept varied heterocyclic precursors (thiophenes, furans, pyrroles), extension of Stockigt 2012 work
"Interrupted" Pictet-Spengler reactions, perhaps to start cascade synthesis
Merge Denmark and Leighton work, see if "base-assisted silicon catalysis" might turn over the PS
Lewis acids for Friedel-Crafts-type reactions: http://www.sciencedirect.com/science/article/pii/S0040402009009302
PS of non-activated phenethylamine with aldehydes to give THIQs: One-step preparation of 1-substituted tetrahydroisoquinolines via the Pictet–Spengler reaction using zeolite catalysts, Adrienn Hegedus and Zoltan Hell, TetLett 2004 45, 8553–8555. DOI: 10.1016/j.tetlet.2004.09.097 Paper
PS of non-activated phenethylamine to give THIQ (clay): Pictet-Spengler condensation reactions catalyzed by a recyclable H+-montmorillonite as a heterogeneous Brønsted acid, DOI: 10.1007/s11426-010-0073-4
Achiral LA-catalysed PS to give THIQs: Catalytic Pictet-Spengler reactions using Yb(OTf)3, Manabe, K., D. Nobutou, et al. Bioorganic & Medicinal Chemistry 2005 13(17): 5154-5158. DOI: 10.1016/j.bmc.2005.05.018 Paper
- Development of the Pictet-Spengler Reaction Catalyzed by AuCl3/AgOTf, S. W. Youn, The Journal of Organic Chemistry 2006 71(6), 2521-2523. DOI: 10.1021/jo0524775 Paper
- Calcium-Promoted Pictet-Spengler Reactions of Ketones and Aldehydes, M. J. V. Eynden, K. Kunchithapatham, J. P. Stambuli, Journal of Organic Chemistry 2010, 75, 8542. DOI: 10.1021/jo1019283 Paper
Conclusions, and what's needed in this field
We will write this section last. Conclusions to address advantages and disadvantages of known systems, wrt temp, recoverability, ee, substrate scope.
Currently no examples of catalytic, asymmetric oxa-PS reaction? Review of oxo-PS is Larghi 2011, and has no cat. enantioselective examples
There might be, but if so, they're covered under different names...like Prins cyclizations, Friedel-Crafts, or anomeric (sugar) arylation. It might require a lot of digging! - MAT
Design of "mini-proteins" (think Marcey Waters, Manfred Reetz, or Scott Miller) to accomplish PS reaction in non-biological environment
Substrate limitations: all known examples are based on tryptamine. No examples with original PS system of an activated benzene ring. No examples with other Ar rings such as furans, thiophenes? One with pyrroles (Jacobsen). One iso-PS reaction (Jacobsen). Recent example of cat asymm on ISQ ring system - first reported: Monitoring On-Chip Pictet-Spengler Reactions by Integrated Analytical Separation and Label-Free Time-Resolved Fluorescence, Ohla Stefan; Beyreiss Reinhild; Fritzsche Stefanie; et al. Source: CHEMISTRY-A EUROPEAN JOURNAL 2012, Volume: 18, 1240-1246 DOI: paper
References
Papers discussed in the review should ONLY be listed here when the summary of the science in the review is complete. The papers may be found in full at the Mendeley page)
Introduction:
- Ueber die Bildung von Isochinolin-derivaten durch Einwirkung von Methylal auf Phenyl-aethylamin, Phenyl-alanin und Tyronsin, A. Pictet and T. Spengler, Ber. Dtsch. Chem. Ges. 1911, 44, 2030-2036. Paper
- Tatsui, G. J. Pharm. Soc. Jpn. 1928, 48, 92 (may be 453-459). Need to find this paper!
- The Pictet-Spengler Condensation: A New Direction for an Old Reaction, E. D. Cox and J. M. Cook, Chem. Rev. 1995, 95, 1797- 1842 Paper
- Asymmetric Synthesis of Isoquinoline Alkaloids, M. Chrzanowska and M. D. Rozwadowska, Chem. Rev. 2004, 104, 3341-3370 Paper
- The Pictet-Spengler Reaction in Nature and in Organic Chemistry, J. Stoeckigt, A. P. Antonchick, F. Wu and H. Waldmann, Angew. Chem. Int. Ed. 2011, 50, 8538-8564 Paper
- The Pictet-Spengler Synthesis of Tetrahydroisoquinolines and Related Compounds, W. M. Whaley and T. R. Govindachari, Organic Reactions 1951, 6, 151-190 Paper
- Enantiospecific Total Synthesis of the Ajmaline Related Alkaloids (-)-Suaveoline, (-)-Raumacline, and (-)-Nb-Methylraumacline, X. Fu and J.M. Cook, J. Am. Chem. Soc. 1992, 114, 6910-6912 Paper
- Resolution of etodolac and anti-inflammatory and prostaglandin synthetase inhibiting properties of the enantiomers, C. A. Demerson, L. G. Humber, N. A. Abraham, G. Schilling, R. R. Martel and C. Pace-Asciak, J. Med. Chem 1983, 26, 1778-1780. Paper
- The Pictet-Spengler Reaction: Efficient Carbon-carbon Bond Forming Reaction in Heterocyclic Synthesis, S. W. Youn, Org. Prep. Proc. Int. 2006, 38, 505-591 [Paper]
- Taste, A New Incentive to Switch to (R)-Praziquantel in Schistosomiasis Treatment, T. Meyer, H. Sekljic, S. Fuchs, H. Bothe, D. Schollmeyer, C. Miculka, PLoS Negl Trop Dis 2009, 3, e357 Paper
- Spiroindolones, a Potent Compound Class for the Treatment of Malaria, M. Rottmann, C. McNamara, B. K. S. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. E. González-Páez, S. B. Lakshminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler, T. T. Diagana, Science 2010, 329, 1175-1180 Paper
- The Total Synthesis of Enantiopure Phalarine via a Stereospecific Pictet-Spengler Reaction: Traceless Transfer of Chirality from L-tryptophane, J.J. Trzupek, D. Lee, B.M. Crowley, V.M. Marathias, S.J. Danishefsky, J. Am. Chem. Soc. 2010, 132, 8506-8512 Paper
- Synthesis of Oxacycles Employing the Oxa-Pictet-Spengler Reaction: Recent Developments and New Prospects, E. L. Larghi and T. S. Kaufman, Eur. J. Org. Chem. 2011, 5195-5231 Paper
Diastereoselective Intro Section:
- Enantiospecific Formation of Trans 1,3-Disubstituted Tetrahydro-β-carbolines by the Pictet-Spengler Reaction and Conversion of Cis Diastereomers into Their Trans Counterparts by Scission of the C-1/N-2 Bond, E. D. Cox, L. K. Hamaker, J. Li, P. Yu, K. M. Czerwinski, L. Deng, D. W. Bennett and J. M. Cook, J. Org. Chem. 1997, 62, 44-61. Paper
- The Intermolecular Pictet-Spengler Condensation with Chiral Carbonyl Derivatives in the Stereoselective Syntheses of Optically-active Isoquinoline and Indole Alkaloids, E. L. Larghi, M. Amongero, A. B. J. Bracca and T. S. Kaufman, ARKIVOC 2005 (xii) 98-153. [Link here: http://www.arkat-usa.org/arkivoc-journal/browse-arkivoc/2005/12/]
Lewis Acid Intro Section:
- A New Evidence for the Presence of a Spiroindolenium Species in the Pictet-Spengler Reaction, T. Kawate, M. Nakagawa, K. Ogata, and T. Hino, Heterocycles, 1992, 33, 801-811. [Need DOI]
- A New Chiral BLA Promoter for Asymmetric Aza Diels-Alder and Aldol-Type Reactions of Imines, K. Ishihara, M. Miyata, K. Hattori, T. Tada and H. Yamamoto, J. Am. Chem. Soc. 1994, 116, 10520–10524. Paper
- Enantioselective Asymmetric Pictet-Spengler Reaction Catalyzed by Diisopinocampheylchloroborane, T. Kawate, H. Yamada, T. Soe and M. Nakagawa, Tetrahedron: Asymmetry 1996, 7, 1249-1252 Paper
- Chiral Lewis Acid-mediated Enantioselective Pictet-Spengler Reaction of N-b-Hydroxytryptamine with Aldehydes, H. Yamada, T. Kawate, M. Matsumizu, A. Nishida, K. Yamaguchi and M. Nakagawa, J. Org. Chem. 1998, 63, 6348-6354. Paper
- Highly Enantioselective Pictet–Spengler Reactions with α-Ketoamide- Derived Ketimines: Access to an Unusual Class of Quaternary α-Amino Amides, F. R. Bou-Hamdan and J. L. Leighton, Angew. Chem. Int. Ed. 2009, 48, 2403-2406 Paper
- Powerful and Versatile Silicon Lewis Acids for Asymmetric Chemical Synthesis, J. L. Leighton, Aldrichimica Acta 2010, 43, 3-12 [Paper available at http://www.sigmaaldrich.com/chemistry/chemical-synthesis/learning-center/aldrichimica-acta.html]
- Direct and Highly Enantioselective Iso-Pictet Spengler Reactions with α-Ketoamides: Access to Underexplored Indole Core Structures, Heike Schönherr and James L. Leighton, Org. Lett. 2012, 14, 2610-2613 Paper
Brønsted Section:
- Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid, T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Angew. Chem. Int. Ed. 2004, 43, 1566-1568. Paper
- Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation, D. Uraguchi and M. Terada, J. Am. Chem. Soc. 2004, 126, 5356-5357. Paper
- Catalytic Asymmetric Pictet-Spengler Reaction, J. Seayad, A. M. Seayad and B. List, J. Am. Chem. Soc. 2006, 128, 1086-1087. Paper
- Catalytic Asymmetric Pictet-Spengler Reactions via Sulfenyliminium Ions, M. J. Wanner, R. N. S. van der Haas, K. R. de Cuba, J. H. van Maarseveen and H. Hiemstra, Angew. Chem. Int. Ed. 2007, 46, 7485-7487. Paper
- Enantioselective BINOL-phosphoric Acid Catalyzed Pictet-Spengler Reactions of N-benzyltryptamine, N. V. Sewgobind, M. J. Wanner, S. Ingemann, R. de Gelder, J. H. van Maarseveen and H. Hiemstra, J. Org. Chem. 2008, 73, 6405-6408. Paper
- Organocatalytic Enantioselective Total Synthesis of (-)-Arboricine, M. J. Wanner, R. N. A. Boots, B. Eradus, R. de Gelder, J. H. van Maarseveen and H. Hiemstra, Org. Lett. 2009, 11, 2579-2581. Paper
- Enantioselective Brønsted Acid-Catalyzed N-Acyliminium Cyclization Cascades, M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc. 2009, 131, 10796-10797. [1]
- Chiral Phosphoric Acids as Versatile Catalysts for Enantioselective Carbon-Carbon Bond Forming Reactions, M. Terada, Bull. Chem. Soc. Jpn. 2010, 83, 101-119. Paper
- Direct Enantioselective Brønsted Acid Catalyzed N-Acyliminium Cyclization Cascades of Tryptamines and Ketoacids, C. A. Holloway, M. E. Muratore, R. I. Storer and D. J. Dixon, Org. Lett., 2010, 12, 4720-4723 Paper
- Total Synthesis of (+)-Yohimbine via an Enantioselective Organocatalytic Pictet-Spengler Reaction, B. Herle, M. J. Wanner, J. H. van Maarseveen and H. Hiemstra, J. Org. Chem. 2011, 76, 8907-8912. Paper
- Enantioselective Syntheses of Corynanthe Alkaloids by Chiral Brønsted Acid and Palladium Catalysis, M. J. Wanner, E. Claveau, J. H. van Maarseveen and H. Hiemstra, Chem. Eur. J. 2011, 17, 13680-13683. Paper
- Enantioselective Pictet-Spengler Reactions of Isatins for the Synthesis of Spiroindolones, J. J. Badillo, A. Silva-Garcia, B. H. Shupe, J. C. Fettinger and A. K. Franz, Tetrahedron Lett. 2011, 52, 5550-5553. Paper
Organocatalysis Section:
- Highly Enantioselective Catalytic Acyl-Pictet-Spengler Reactions, M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc. 2004, 126, 10558-10559. Paper
- Small-Molecule H-Bond Donors in Asymmetric Catalysis, A. G. Doyle and E. N. Jacobsen, Chem. Rev. 2007, 107, 5713-5743. Link? <-- (MHT, Jan 22) - has this paper been read and relevant mechanistic ideas incorporated?
- Enantioselective Pictet-Spengler-Type Cyclizations of Hydroxylactams: H-Bond Donor Catalysis by Anion Binding, I. T. Raheem, P. S. Thiara, E. A. Peterson and E. N. Jacobsen, J. Am. Chem. Soc. 2007, 129, 13404-page?. Paper
- Catalytic Asymmetric Total Synthesis of (+)-Yohimbine, D. J. Mergott, S. J. Zuend and E. N. Jacobsen, Org. Lett. 2008, 10, 745-748. Paper
- Weak Brønsted Acid-thiourea Co-catalysis: Enantioselective, Catalytic Protio-Pictet-Spengler Reactions, R. S. Klausen and E. N. Jacobsen, Org. Lett. 2009, 11, 887-890. Paper
- Thiourea-Catalyzed Enantioselective Iso-Pictet-Spengler Reactions, Y. Lee, R. S. Klausen and E. N. Jacobsen, Org. Lett. 2011, 13, 5564-5567. Paper
Enzyme Section:
- Strictosidine Synthase: Mechanism of a Pictet-Spengler Catalyzing Enzyme, J.J. Maresh, L-A. Giddings, A. Friedrich, E.A. Loris, S. Panjikar, B.L. Trout, J. Stockigt, B. Peters, S.E. O'Connor. J. Am. Chem. Soc. 2008, 130, 710-723.
- Integrating carbon-halogen bind formation into medicinal plant metabolism, W. Runguphan, X. Qu, S.E. O'Connor. Nature 2010, 468, 461-467.
- Biocatalytic Asymmetric Formation of Tetrahydro-B-carbolines, P. Bernhardt, A.S. Usera, and S.E. O'Connor, Tetrahedron Lett. 2010, 51, 4400-4402.
- Scaffold Tailoring by a Newly Detected Pictet-Spenglerase Activity of Strictosidine Synthase: From the Common Tryptoline Skeleton to the Rare Piperazino-indole Framework, F. Wu, H. Zhu, L. Sun, C. Rajendran, M. Wang, X. Ren, S. Panjikar, A. Cherkasov, H. Zou, and J. Stöckigt, J. Am. Chem. Soc. 2012, 134, 1498-1500. Paper
Papers we're not including, and why (arranged by date)
Insert details of papers here if they are not being used, and explain why.
- Asymmetric Pictet-Spengler Reactions Employing N,N-Phthaloyl Amino Acids as Chiral Auxiliary Groups, H. Waldmann, G. Schmidt, H. Henke and M. Burkard, Angew. Chem., Int. Ed. Engl. 1995, 34, 2402-2403. Paper - Diastereoselective. Use of stoichiometric chiral auxiliaries.
- Asymmetric Control in Pictet-Spengler Reaction by Means of N-Protected Amino Acids as Chiral Auxiliary Groups, G. Schmidt, H. Waldmann, H. Henke and M. Burkard, Chem. Eur. J. 1996, 2, 1566-1571. Paper - Diastereoselective. Use of stoichiometric chiral auxiliaries.
- Enantiopure Tetrahydro-β-carbolines via Pictet−Spengler Reactions with N-Sulfinyl Tryptamines, C. Gremmen, B. Willemse, M. J. Wanner, G.-J. Koomen, Org. Lett. 2000, 2, 1955-1958. [Paper]- Use of chiral auxiliary.
- Enantiopure tetrahydroisoquinolines via N-sulfinyl Pictet–Spengler reactions, C. Gremmen, M. J. Wanner, G Koomen, G.-J. Tetrahedron Lett. 2001, 42, 8885-8888. Paper - Use of chiral auxiliary and excess acid.
- Enantioselective Addition of Amines to Ketenes Catalyzed by a Planar-Chiral Derivative of PPY: Possible Intervention of Chiral Brønsted-Acid Catalysis, B. L. Hodous, G. C. Fu, J. Am. Chem. Soc., 2002, 124, 10006-10007, Paper - Reaction of pyrroles with ketenes catalysed by a PPY planar-chiral 4-(pyrrolidino)pyridine (PPY), which is Fe π-bonded complex. Not Pictet-Spengler.
- A Chiral Acrylate Equivalent for Metal-Free Diels-Alder Reactions: endo-2-Acryloylisoborneol, C. Palomo, M. Oiarbide, J. M. Garcia, A. Gonzalez, A. Lecumberri, A. Linden, J. Am. Chem. Soc. 2002, 124, 10288 Paper - Not relevant. Not chiral acid, use of chiral aux. in Diels-Alder.
- General Approach for the Synthesis of Sarpagine Indole Alkaloids. Enantiospecific Total Synthesis of (+)-Vellosimine, (+)-Normacusine B, (-)-Alkaloid Q3, (-)-Panarine, (+)-Na-Methylvellosimine, and (+)-Na-Methyl-16-epipericyclivine, J. Yu, T. Wang, X. Liu, J. Deschamps, J. Flippen-Anderson, X. Liao and J. M. Cook, J. Org. Chem. 2003, 68, 7565-7581. Paper - diastereoselective
- Organocatalytic Asymmetric Aza-Friedel-Crafts Alkylation of Furan, D. Uraguchi, K. Sorimachi and M. Terada, J. Am. Chem. Soc. 2004, 126, 11804-11805. Paper - Maybe include, but non-PS, simple extension of previous paper.
- Organocatalytic Asymmetric Direct Alkylation of α-Diazoester via C−H Bond Cleavage, D. Uraguchi, K. Sorimachi and M. Terada, J. Am. Chem. Soc. 2005, 127, 9360-9361. Paper - extension to a system too far removed from PS reaction.
- Chiral Brønsted Acid Catalyzed Enantioselective Hydrophosphonylation of Imines: Asymmetric Synthesis of α-Amino Phosphonates, T. Akiyama, H. Morita, J. Itoh and K. Fuchibe, Org. Lett. 2005, 7, 2583–2585. Paper - simple extension to other nucleophile.
- Stereocontrolled Total Synthesis of (-)-Eudistomin C, T. Yamashita, N. Kawai, H. Tokuyama and T. Fukuyama, J. Am. Chem. Soc. 2005, 127, 15038-15039. Paper - Diastereoselective.
- An Improved Total Synthesis of (+)-Macroline and Alstonerine as Well as the Formal Total Synthesis of (-)-Talcarpine and (-)-Anhydromacrosalhine-methine, X. Liao, H. Zhou, J. Yu and J. M. Cook, J. Org. Chem. 2006, 71, 8884-8890. Paper - presumed diastereoselective, but relevant chemistry is actually in J. Org. Chem. 2000, 65, 3173.
- Total Synthesis of the Opioid Agonistic Indole Alkaloid Mitragynine and the First Total Syntheses of 9-Methoxygeissoschizol and 9-Methoxy-Nb-methylgeissoschizol, J. Ma, W. Yin, H. Zhou and J. M. Cook, Org. Lett. 2007, 9, 3491-3494. Paper - diastereoselective.
- The Asymmetric Pictet-Spengler Reaction, M. Lorenz, M. L. van Linn and J. M. Cook, Curr. Org. Synth. 2010, 7, 189-223 Paper - this is a recent review, but persistently difficult to get hold of, so I suggest we do not include a link it. Waldmann's is I think more available and covers relevant material.
- K. Pulka, Curr. Opin. Drug Discov. Devel. 2010, 13, 669–684 - referenced from Franz 2011 but not easily available (MHT Feb 2) - ignore?
- Synthesis of beta-Carbolines from Aldehydes and Ketones via the alpha-Siloxy alpha,beta-Unsaturated Esters, S. He, Z. Lai, D. X. Yang et al. Tetrahedron Lett, 2010, 51, 4361-4364 paper - syntheses are rac.
- Intra- and Intermolecular Oxa-Pictet–Spengler Cyclization Strategy for the Enantioselective Synthesis of Deoxy Analogues of (+)-Nanomycin A Methyl Ester, (+)-Eleutherin, (+)-Allo-Eleutherin, and (+)-Thysanone, R. T. Sawant, S. G. Jadhav and S. B. Waghmode, Eur. J. Org. Chem. 2010, 4442-4449 Paper - diastereoselective
- Electrochemical Deallylation of alpha-Allyl Cyclic Amines and Synthesis of Optically Active Quaternary Cyclic Amino Acids, P. G. Kirira, M. Kuriyama and O. Onomura, Chem. Eur. J. 2010, 16, 3970-3982 paper - electrochemical generation of iminium ions - not relevant.
- Total Synthesis of (–)-Corynantheidine by Nickel-Catalyzed Carboxylative Cyclization of Enynes, T Mizuno, Y. Oonishi, M. Takimoto, and Y. Sato, Eur. J. Org. Chem. 2011, 2606-2609. Paper - diastereoselective PS as part of longer synthesis.
- Highly Enantioselective Mannich Reactions with alpha-Aryl Silyl Ketene Acetals and Imines, G. T. Notte, J. M. B. Vu and J. L. Leighton, Org. Lett. 2011, 13, 816-818 paper - adaptation of Leighton's methodology to Mannich reactions.
- Catalytic Asymmetric Synthesis of 1,1-Disubstituted Tetrahydro-beta-carbolines by Phase-transfer Catalyzed Alkylations, S. Shirakawa, K. Liu, H. Ito et al. Chem. Commun. 2011, 47, 1515-1517 paper - not PS, not relevant
- Chiral Phosphoric Acid-catalysed Friedel-Crafts Alkylation Reaction of Indoles with Racemic Spiro Indolin-3-ones, Q. Yin and S.-L. You. Chem. Sci. 2011, 2, 1344-1348 paper - non-PS, not relevant - chiral phos acid + indole + imine.
- A Straightforward Synthesis of N-Monosubstituted Alpha-keto Amides via Aerobic Benzylic Oxidation of Amides, J. Shao, X. Huang, S. Wang et al. Tetrahedron 2012, 68, 573-579 paper - not relevant - just synthesis of ketoamides.
- Lipase-Catalyzed Kinetic Resolution of 2-Phenylethanol Derivatives and Chiral Oxa-Pictet–Spengler Reaction as the Key Steps in the Synthesis of Enantiomerically Pure Tricyclic Amines, C. Ketterer and B. Wünsch, Eur. J. Org. Chem. 2012, 2428. [2] - diastereoselective
